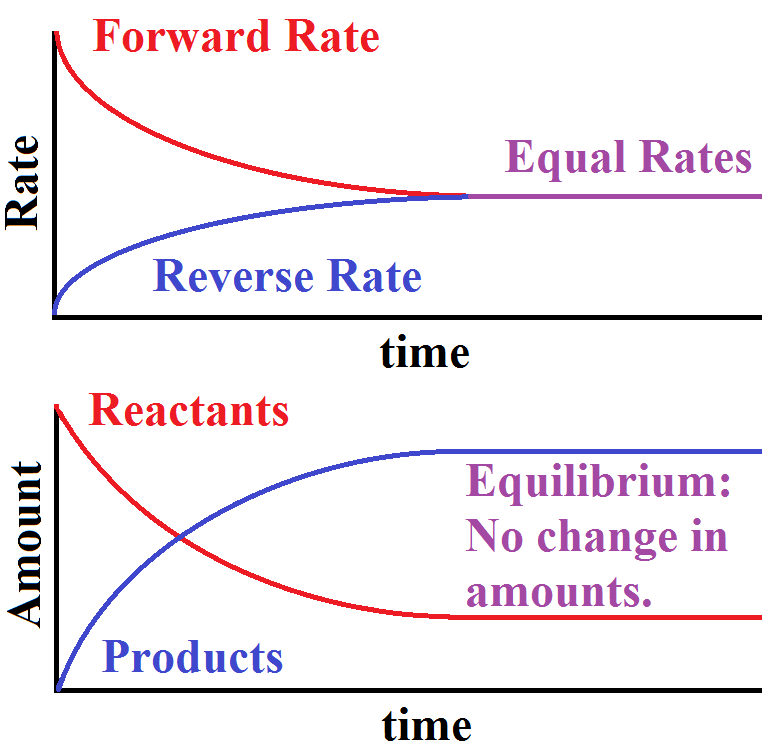
How Is Chemical Equilibrium Used In Real Life . despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. describe examples of systems involving two (or more) simultaneous chemical equilibria; in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. It is possible to predict the. When a chemical reaction takes place in a container which prevents the entry or escape of any of the.
from www.online-sciences.com
chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. When a chemical reaction takes place in a container which prevents the entry or escape of any of the. It is possible to predict the. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. describe examples of systems involving two (or more) simultaneous chemical equilibria;
Chemical Equilibrium, Chemical reactions types, complete reactions and
How Is Chemical Equilibrium Used In Real Life When a chemical reaction takes place in a container which prevents the entry or escape of any of the. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. describe examples of systems involving two (or more) simultaneous chemical equilibria; When a chemical reaction takes place in a container which prevents the entry or escape of any of the. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. It is possible to predict the. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations.
From www.chemicals.co.uk
Chemistry A Level Revision Equilibrium How Is Chemical Equilibrium Used In Real Life It is possible to predict the. describe examples of systems involving two (or more) simultaneous chemical equilibria; chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. When a. How Is Chemical Equilibrium Used In Real Life.
From www.youtube.com
Equilibrium Made Easy How to Solve Chemical Equilibrium Problems YouTube How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. in. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT 16 Chemical Equilibrium PowerPoint Presentation, free download How Is Chemical Equilibrium Used In Real Life It is possible to predict the. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. despite the challenges involved in addressing the subject of equilibrium, the. How Is Chemical Equilibrium Used In Real Life.
From www.chemicals.co.uk
A Level Chemistry Physical Chemistry Chemical Equilibria How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. describe examples of systems involving two (or more) simultaneous chemical equilibria; It is possible to predict the. When a chemical reaction takes. How Is Chemical Equilibrium Used In Real Life.
From www.len.com.ng
Chemical Equilibrium Dynamic Equilibrium in Chemistry How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. describe examples of systems involving two (or more) simultaneous chemical equilibria; When a chemical reaction takes place in. How Is Chemical Equilibrium Used In Real Life.
From www.chemistrylearner.com
Chemical Equilibrium Definition, Principles, and Examples How Is Chemical Equilibrium Used In Real Life It is possible to predict the. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. When a. How Is Chemical Equilibrium Used In Real Life.
From www.chemistrylearner.com
Chemical Equilibrium Definition, Principles, and Examples How Is Chemical Equilibrium Used In Real Life this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. describe examples of systems involving two (or more) simultaneous chemical equilibria; When a chemical reaction takes place in a container which prevents the. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. describe examples of systems involving two (or more) simultaneous chemical equilibria; in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. It is possible to predict the. this dynamic state,. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. When a chemical reaction takes place in a container which prevents the entry or escape of any of the. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. despite the challenges involved. How Is Chemical Equilibrium Used In Real Life.
From gamesmartz.com
Law Of Chemical Equilibrium Definition Easy to Understand How Is Chemical Equilibrium Used In Real Life It is possible to predict the. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. When a chemical reaction takes place in a container which prevents the. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. It is possible. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. When a chemical. How Is Chemical Equilibrium Used In Real Life.
From alevelchemistry.co.uk
Chemical Equilibrium ALevel Chemistry Revision Notes How Is Chemical Equilibrium Used In Real Life It is possible to predict the. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. describe examples of systems involving two (or more) simultaneous chemical equilibria; this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium.. How Is Chemical Equilibrium Used In Real Life.
From www.youtube.com
Chemical equilibrium Chemistry YouTube How Is Chemical Equilibrium Used In Real Life It is possible to predict the. describe examples of systems involving two (or more) simultaneous chemical equilibria; despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. When a chemical reaction takes. How Is Chemical Equilibrium Used In Real Life.
From www.bartleby.com
Chemical Equilibrium bartleby How Is Chemical Equilibrium Used In Real Life this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. When a chemical reaction takes place in a container which prevents the entry or escape of any of the. describe examples of systems involving two (or more) simultaneous chemical equilibria; in a chemical reaction, chemical equilibrium is the state. How Is Chemical Equilibrium Used In Real Life.
From thechemistrynotes.com
Chemical Equilibrium Definition, Types, Importance, and Examples How Is Chemical Equilibrium Used In Real Life When a chemical reaction takes place in a container which prevents the entry or escape of any of the. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. this. How Is Chemical Equilibrium Used In Real Life.
From courses.lumenlearning.com
13.1 Chemical Equilibrium General College Chemistry II How Is Chemical Equilibrium Used In Real Life It is possible to predict the. When a chemical reaction takes place in a container which prevents the entry or escape of any of the. describe examples of systems involving two (or more) simultaneous chemical equilibria; in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. this. How Is Chemical Equilibrium Used In Real Life.
From www.thoughtco.com
Chemical Equilibrium in Chemical Reactions How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of. How Is Chemical Equilibrium Used In Real Life.
From www.youtube.com
Chemical Equilibrium Using Gibbs Minimization Example YouTube How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. describe examples of systems involving two (or more) simultaneous chemical equilibria; this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. It is possible to predict the. chemical equilibrium, condition in. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. It is possible to predict the. in a chemical reaction, chemical equilibrium is the state in which both. How Is Chemical Equilibrium Used In Real Life.
From www.youtube.com
What is Chemical Equilibrium Explain with Example Class 10 Chemistry How Is Chemical Equilibrium Used In Real Life chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. describe examples of systems involving two (or more) simultaneous chemical equilibria; in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. chemical reactions eventually. How Is Chemical Equilibrium Used In Real Life.
From courses.lumenlearning.com
Chemical Equilibria Chemistry How Is Chemical Equilibrium Used In Real Life chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be.. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free How Is Chemical Equilibrium Used In Real Life describe examples of systems involving two (or more) simultaneous chemical equilibria; chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. in a chemical reaction, chemical equilibrium is. How Is Chemical Equilibrium Used In Real Life.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and How Is Chemical Equilibrium Used In Real Life in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. It is possible to predict the. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. chemical equilibrium, condition in the course of a reversible chemical reaction in which no. How Is Chemical Equilibrium Used In Real Life.
From www.youtube.com
Chemical equilibrium with real examples YouTube How Is Chemical Equilibrium Used In Real Life this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. It is possible to predict the. despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be.. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. describe examples of systems involving two (or more) simultaneous chemical equilibria; When a chemical reaction takes place in a container which prevents the. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. describe examples of systems involving two (or more) simultaneous chemical equilibria; chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. When a chemical reaction takes place in a container which prevents the. How Is Chemical Equilibrium Used In Real Life.
From www.scribd.com
2. Chemical Equilibrium Chemical Equilibrium Mechanics How Is Chemical Equilibrium Used In Real Life this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. When a chemical reaction takes place in a container which prevents the entry or escape of any of the. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. It is possible to predict the. When a chemical reaction takes place in a container which prevents the entry or escape of any of the. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's.. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life describe examples of systems involving two (or more) simultaneous chemical equilibria; When a chemical reaction takes place in a container which prevents the entry or escape of any of the. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. chemical equilibrium, condition in the course of a reversible chemical reaction. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM Chapter 16 PowerPoint Presentation, free How Is Chemical Equilibrium Used In Real Life It is possible to predict the. chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. describe examples of systems involving two (or more) simultaneous chemical equilibria; this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. despite the challenges involved in. How Is Chemical Equilibrium Used In Real Life.
From study.com
Dynamic & Chemical Equilibrium Definition & Examples Lesson How Is Chemical Equilibrium Used In Real Life in a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations. It is possible to predict the. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. despite the challenges involved in addressing the subject of equilibrium, the results of. How Is Chemical Equilibrium Used In Real Life.
From www.shutterstock.com
Chemical Equilibrium Infographic Diagram Showing Lab Stock Illustration How Is Chemical Equilibrium Used In Real Life chemical reactions eventually reach equilibrium, a point at which forward and reverse reactions balance each other's. It is possible to predict the. chemical equilibrium, condition in the course of a reversible chemical reaction in which no net change in the amounts of reactants and. this dynamic state, in which the concentrations of reactants and products remains constant,. How Is Chemical Equilibrium Used In Real Life.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID How Is Chemical Equilibrium Used In Real Life despite the challenges involved in addressing the subject of equilibrium, the results of chemical equilibrium can be. describe examples of systems involving two (or more) simultaneous chemical equilibria; It is possible to predict the. this dynamic state, in which the concentrations of reactants and products remains constant, is referred to as equilibrium. in a chemical reaction,. How Is Chemical Equilibrium Used In Real Life.