Salt And Melting Point Of Ice . This phenomenon is called freezing point depression. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the water's freezing point via freezing point depression. But how does salt do it? When you mix salt onto that layer, it slowly lowers its melting point. Why does salt melt ice. The salt has to dissolve into its ions in order to work. The more surface area salt can cover, the better the chances for melting ice. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. First, it’s important to understand a bit about h 2 o in the winter. Melting is endothermic, so it lowers the temperature. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. Salt only helps if there is a little bit of liquid water available.
from 88guru.com
Salt lowers the water's freezing point via freezing point depression. First, it’s important to understand a bit about h 2 o in the winter. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Melting is endothermic, so it lowers the temperature. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. This phenomenon is called freezing point depression. Salt makes ice colder because the salt prevents melted water from freezing. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. But how does salt do it?
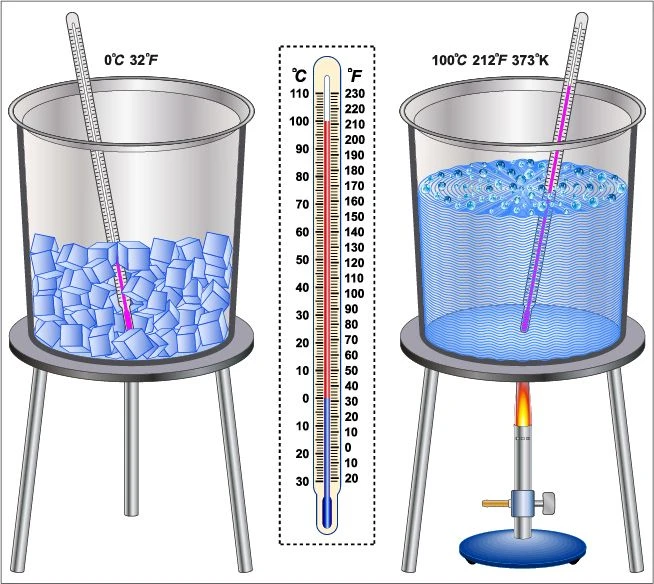
Melting Point of Ice and Boiling Point of Water 88Guru
Salt And Melting Point Of Ice When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. First, it’s important to understand a bit about h 2 o in the winter. When you mix salt onto that layer, it slowly lowers its melting point. When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. Salt makes ice colder because the salt prevents melted water from freezing. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. But how does salt do it? Salt only helps if there is a little bit of liquid water available. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. Salt lowers the water's freezing point via freezing point depression. Melting is endothermic, so it lowers the temperature. Why does salt melt ice. The salt has to dissolve into its ions in order to work. The more surface area salt can cover, the better the chances for melting ice.
From fphoto.photoshelter.com
science chemistry melting point Fundamental Photographs The Art of Salt And Melting Point Of Ice Salt makes ice colder because the salt prevents melted water from freezing. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. Salt lowers the water's freezing point via freezing point depression. When you mix salt onto that layer, it slowly lowers its melting point. When salt is sprinkled over. Salt And Melting Point Of Ice.
From kimberlyscottscience.blogspot.com
Science It's All Elementary Exploring the Effects of Salt on Ice with Salt And Melting Point Of Ice The more surface area salt can cover, the better the chances for melting ice. Salt makes ice colder because the salt prevents melted water from freezing. Salt lowers the water's freezing point via freezing point depression. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. When you mix salt onto. Salt And Melting Point Of Ice.
From www.youtube.com
what's the point of SALT in making Ice Cream YouTube Salt And Melting Point Of Ice But how does salt do it? Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. Melting is endothermic, so it lowers the temperature. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. First, it’s important to. Salt And Melting Point Of Ice.
From www.scientificamerican.com
Salt Doesn't Melt IceHere's How It Makes Winter Streets Safer Salt And Melting Point Of Ice The salt has to dissolve into its ions in order to work. The more surface area salt can cover, the better the chances for melting ice. But how does salt do it? This phenomenon is called freezing point depression. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. At 0 °c, ice melts and freezes. Salt And Melting Point Of Ice.
From www.numerade.com
A student is investigating the affect of different salts on melting Salt And Melting Point Of Ice Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. But how does salt do. Salt And Melting Point Of Ice.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC Salt And Melting Point Of Ice Salt only helps if there is a little bit of liquid water available. When you mix salt onto that layer, it slowly lowers its melting point. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Melting is endothermic, so it lowers the temperature. But how does salt do it? Salt makes ice colder because the. Salt And Melting Point Of Ice.
From answerbun.com
Melting ice under pressure Physics Salt And Melting Point Of Ice This phenomenon is called freezing point depression. But how does salt do it? Melting is endothermic, so it lowers the temperature. The salt has to dissolve into its ions in order to work. When you mix salt onto that layer, it slowly lowers its melting point. More than 20 million tons of salt are used every year to melt snow. Salt And Melting Point Of Ice.
From huntingwaterfalls.com
Does Salt Make Ice Colder? If So, Why? Salt And Melting Point Of Ice The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. This phenomenon is called freezing point depression. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. More than 20 million tons of salt are. Salt And Melting Point Of Ice.
From frugalfun4boys.com
Melting Ice Science Experiments {Fun!} Frugal Fun For Boys and Girls Salt And Melting Point Of Ice This phenomenon is called freezing point depression. The more surface area salt can cover, the better the chances for melting ice. Melting is endothermic, so it lowers the temperature. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Among other processes, the ions from the salt get in the way of water molecules aligning to. Salt And Melting Point Of Ice.
From animalia-life.club
Ice Cube Melting Science Project Salt And Melting Point Of Ice Melting is endothermic, so it lowers the temperature. The more surface area salt can cover, the better the chances for melting ice. Salt only helps if there is a little bit of liquid water available. The salt has to dissolve into its ions in order to work. But how does salt do it? You might have seen salt being sprinkled. Salt And Melting Point Of Ice.
From www.slideserve.com
PPT MELTING AND BOILING PowerPoint Presentation, free download ID Salt And Melting Point Of Ice The salt has to dissolve into its ions in order to work. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Melting is endothermic, so it lowers the temperature. But how does salt do it? When salt is sprinkled over the ice without adding water, the salt will dissolve. Salt And Melting Point Of Ice.
From www.youtube.com
Science Fun Ice Melting With Salt YouTube Salt And Melting Point Of Ice Salt only helps if there is a little bit of liquid water available. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. First, it’s important to understand a bit about h 2 o in the winter. Melting is endothermic, so it lowers the temperature. More than 20 million tons. Salt And Melting Point Of Ice.
From www.worksheetsplanet.com
What is Melting Point Definition of Melting Point Salt And Melting Point Of Ice When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. Salt makes ice colder because the salt prevents melted water from freezing. First, it’s important to understand a bit about h 2 o in the winter. Melting is endothermic, so it lowers the temperature. But. Salt And Melting Point Of Ice.
From www.slideserve.com
PPT Melting Points and Mixed Melting Points PowerPoint Presentation Salt And Melting Point Of Ice More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Why does salt melt ice. The salt has to dissolve into its ions in order to work. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When you mix. Salt And Melting Point Of Ice.
From ian.umces.edu
Experiment Demonstrating Melting Ice Media Library Integration and Salt And Melting Point Of Ice Salt makes ice colder because the salt prevents melted water from freezing. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. First, it’s important to understand a bit about h 2 o in. Salt And Melting Point Of Ice.
From sciencenotes.org
Why Salt Makes Ice Colder How Cold Ice Gets Salt And Melting Point Of Ice When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. Salt makes ice colder because the salt prevents melted water from freezing. First, it’s important to understand a bit about h 2 o in the winter. Melting is endothermic, so it lowers the temperature. Salt only helps if. Salt And Melting Point Of Ice.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice Salt And Melting Point Of Ice Salt lowers the water's freezing point via freezing point depression. First, it’s important to understand a bit about h 2 o in the winter. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. At 0 °c, ice melts and freezes at the same rate, so you don't see ice. Salt And Melting Point Of Ice.
From edp6b2016.blogspot.com
EDP 6B 2016 Cycles Activity 6 Melting & Freezing Salt And Melting Point Of Ice When you mix salt onto that layer, it slowly lowers its melting point. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. Salt lowers the water's freezing point via freezing point depression. Why does salt melt ice. Salt makes ice colder because the salt prevents melted water from freezing. The. Salt And Melting Point Of Ice.
From ar.inspiredpencil.com
Melting Point Of Ice Salt And Melting Point Of Ice You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Salt only helps if there is a little bit of liquid water available. Salt makes ice colder because the salt prevents melted water from freezing. First, it’s important to understand a bit about h 2 o in the winter. Why does salt melt ice. Among other. Salt And Melting Point Of Ice.
From www.youtube.com
Why Does Salt Melt Ice? SCI CODE YouTube Salt And Melting Point Of Ice Salt lowers the water's freezing point via freezing point depression. But how does salt do it? Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice. When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. You might. Salt And Melting Point Of Ice.
From www.themunicipal.com
Minimizing the impact of ice The Municipal Salt And Melting Point Of Ice You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Salt makes ice colder because the salt prevents melted water from freezing. The salt has to dissolve into its ions in order to work. First, it’s important to understand a bit about h 2 o in the winter. Salt lowers the water's freezing point via freezing. Salt And Melting Point Of Ice.
From mommaowlslab.blogspot.com
Momma Owl's Lab Melting Ice with Salt Salt And Melting Point Of Ice Salt only helps if there is a little bit of liquid water available. The more surface area salt can cover, the better the chances for melting ice. This phenomenon is called freezing point depression. The salt has to dissolve into its ions in order to work. Among other processes, the ions from the salt get in the way of water. Salt And Melting Point Of Ice.
From publicaffairsworld.com
what kind of salt is used to melt ice Salt And Melting Point Of Ice More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Salt makes ice colder because the salt prevents melted water from freezing. Salt only helps if there is a little bit of liquid water available. First, it’s important to understand a bit about h 2 o in the winter. When. Salt And Melting Point Of Ice.
From www.learnz.org.nz
LEARNZ Salt And Melting Point Of Ice At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. But how does salt do it? Melting is endothermic, so it lowers the temperature. Salt lowers the water's freezing point via freezing point depression. The salt has to dissolve into its ions in order to work. When salt is sprinkled. Salt And Melting Point Of Ice.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru Salt And Melting Point Of Ice Salt lowers the water's freezing point via freezing point depression. But how does salt do it? Why does salt melt ice. Salt makes ice colder because the salt prevents melted water from freezing. This phenomenon is called freezing point depression. Among other processes, the ions from the salt get in the way of water molecules aligning to crystallize into ice.. Salt And Melting Point Of Ice.
From www.youtube.com
Science Experiments To Find Out The Melting Point Of Ice YouTube Salt And Melting Point Of Ice Melting is endothermic, so it lowers the temperature. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. The salt has to dissolve into its ions in order to work. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. Salt only helps if there is. Salt And Melting Point Of Ice.
From 88guru.com
Melting Point of Ice and Boiling Point of Water 88Guru Salt And Melting Point Of Ice The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. Salt makes ice colder because the salt prevents melted water from freezing. But how does salt do it? More than 20 million tons of salt are used every year to melt snow and ice in. Salt And Melting Point Of Ice.
From sciencing.com
Rock Salt Vs. Table Salt to Melt Ice Sciencing Salt And Melting Point Of Ice The more surface area salt can cover, the better the chances for melting ice. When you mix salt onto that layer, it slowly lowers its melting point. The salt has to dissolve into its ions in order to work. Salt only helps if there is a little bit of liquid water available. Salt makes ice colder because the salt prevents. Salt And Melting Point Of Ice.
From www.youtube.com
Melting point of ice, Melting point of ice with salt and Melting point Salt And Melting Point Of Ice This phenomenon is called freezing point depression. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Melting is endothermic, so it lowers the temperature. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. When salt is sprinkled over the ice without adding water, the. Salt And Melting Point Of Ice.
From saltsmart.org
How Does Salt Melt Snow and Ice? Salt Smart Collaborative Salt And Melting Point Of Ice The more surface area salt can cover, the better the chances for melting ice. Salt makes ice colder because the salt prevents melted water from freezing. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Among other processes, the ions from the salt get in the way of water. Salt And Melting Point Of Ice.
From dengarden.com
The Difference Between Rock Salt and Ice Melt Dengarden Salt And Melting Point Of Ice The salt has to dissolve into its ions in order to work. When you mix salt onto that layer, it slowly lowers its melting point. More than 20 million tons of salt are used every year to melt snow and ice in cold northern regions. The more surface area salt can cover, the better the chances for melting ice. You. Salt And Melting Point Of Ice.
From www.worldatlas.com
Why Does Salt Melt Ice? WorldAtlas Salt And Melting Point Of Ice The more surface area salt can cover, the better the chances for melting ice. When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. At 0 °c, ice melts and freezes at the same rate, so you don't see ice melting at this temperature. Why does salt melt. Salt And Melting Point Of Ice.
From www.nbc15.com
The science behind salt melting ice Salt And Melting Point Of Ice But how does salt do it? Salt only helps if there is a little bit of liquid water available. Why does salt melt ice. The salt has to dissolve into its ions in order to work. Melting is endothermic, so it lowers the temperature. Salt lowers the water's freezing point via freezing point depression. The more surface area salt can. Salt And Melting Point Of Ice.
From www.momto2poshlildivas.com
Mom to 2 Posh Lil Divas What Melts Ice the Fastest? A Handson Science Salt And Melting Point Of Ice First, it’s important to understand a bit about h 2 o in the winter. The salt has to dissolve into its ions in order to work. You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. Melting is endothermic, so it lowers the temperature. When salt is sprinkled over the ice without adding water, the salt. Salt And Melting Point Of Ice.
From mirjamglessmer.com
On melting ice cubes and molecular diffusion of heat Adventures in Salt And Melting Point Of Ice When salt is sprinkled over the ice without adding water, the salt will dissolve in meltwater and have the same effect—it only. The more surface area salt can cover, the better the chances for melting ice. But how does salt do it? You might have seen salt being sprinkled on frozen sidewalks and gutters in winter. More than 20 million. Salt And Melting Point Of Ice.