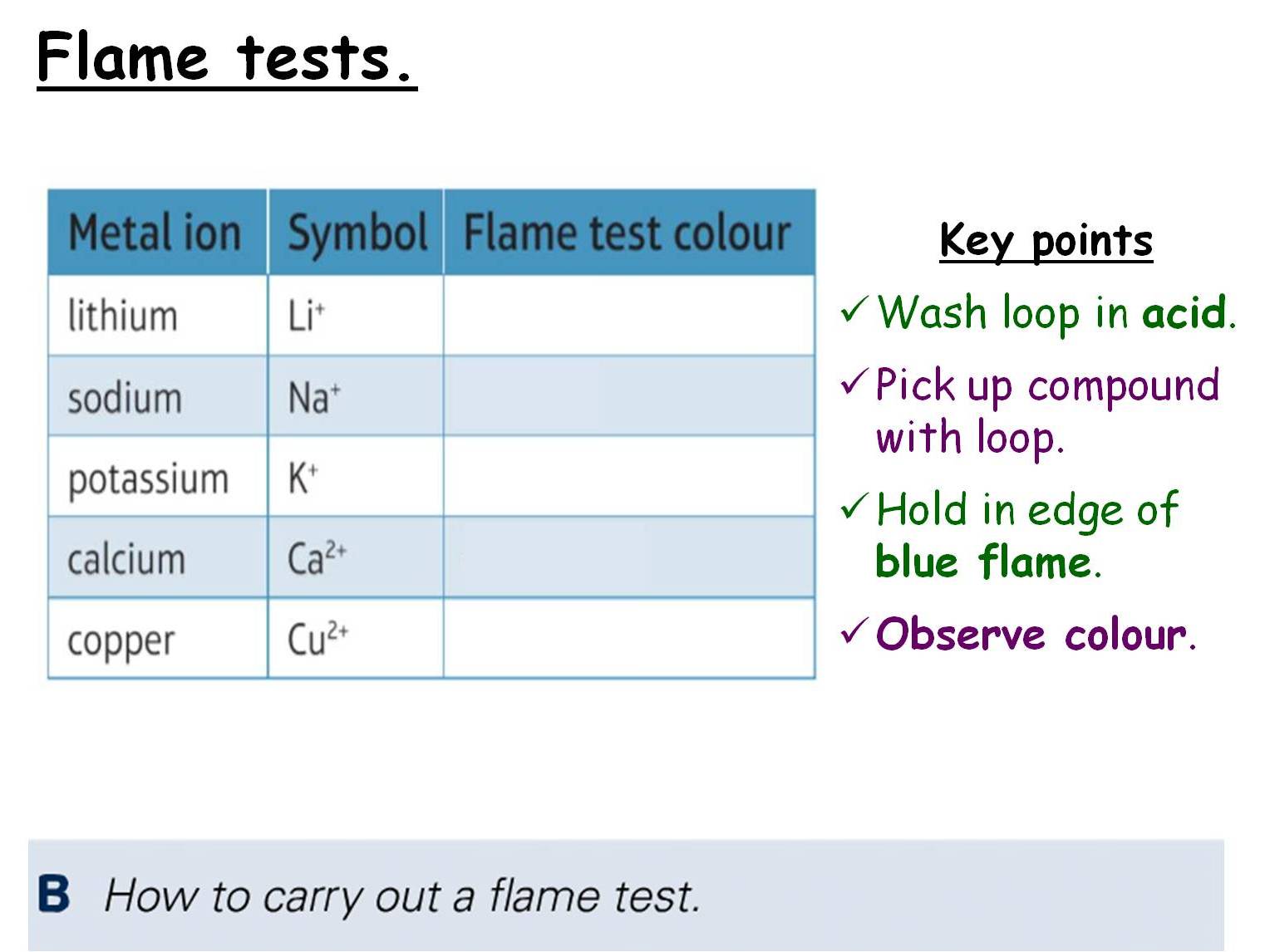
Tests For Inorganic Ions . Test for group 2 ions: Then, test yourself with our. Students will meet chemical tests that. Learn how to separate and analyse chemicals using filtration; Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. A definitive and unique response to a particular substance (ion, molecule or atom). Guide students into testing for negative ions with this practical. Students note their own observations and use their knowledge to discover more. Includes kit list and safety instructions. Instrumental methods of analysis are faster, and more. This experiment involves identifying the cations and anions in various salt solutions. Flame tests and chemical tests are used to detect and identify ions in samples. Chemists often have to identify the composition of unknown substances. A good chemical test needs to give: This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution.
from www.tes.com
This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Then, test yourself with our. Test for group 2 ions: Flame tests and chemical tests are used to detect and identify ions in samples. A definitive and unique response to a particular substance (ion, molecule or atom). Students will meet chemical tests that. A good chemical test needs to give: Instrumental methods of analysis are faster, and more. Learn how to separate and analyse chemicals using filtration; Students note their own observations and use their knowledge to discover more.
OnSpecScience Teaching Resources TES
Tests For Inorganic Ions Students note their own observations and use their knowledge to discover more. Test for group 2 ions: A definitive and unique response to a particular substance (ion, molecule or atom). Then, test yourself with our. Learn how to separate and analyse chemicals using filtration; Students will meet chemical tests that. A good chemical test needs to give: Instrumental methods of analysis are faster, and more. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Guide students into testing for negative ions with this practical. Flame tests and chemical tests are used to detect and identify ions in samples. Includes kit list and safety instructions. Chemists often have to identify the composition of unknown substances. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Students note their own observations and use their knowledge to discover more. This experiment involves identifying the cations and anions in various salt solutions.
From www.savemyexams.co.uk
Testing for Halide Ions (2.3.3) AQA A Level Chemistry Revision Notes Tests For Inorganic Ions Flame tests and chemical tests are used to detect and identify ions in samples. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. This experiment involves identifying the cations and anions in various salt solutions. Then, test yourself with our. Guide students into testing for negative ions with this practical. A good. Tests For Inorganic Ions.
From www.nhbs.com
Detection of Common Ions Environment Friendly Tests NHBS Tests For Inorganic Ions Then, test yourself with our. A good chemical test needs to give: Students will meet chemical tests that. Learn how to separate and analyse chemicals using filtration; A definitive and unique response to a particular substance (ion, molecule or atom). Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Includes kit list. Tests For Inorganic Ions.
From www.chegg.com
Solved ions such as potassium (K+) and chloride Tests For Inorganic Ions Includes kit list and safety instructions. Flame tests and chemical tests are used to detect and identify ions in samples. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Instrumental methods of analysis are faster, and more. A good chemical test needs to give: Test for group 2 ions: Chemists often have. Tests For Inorganic Ions.
From www.flinnsci.com
Ion Names, Formulas and Charges Chart Flinn Scientific Tests For Inorganic Ions Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Learn how to separate and analyse chemicals using filtration; Students note their own observations and use their knowledge to discover more. Includes kit list and safety instructions. Test for group 2 ions: This experiment involves identifying the cations and anions in various salt. Tests For Inorganic Ions.
From www.studocu.com
Ions MS Practice questions Page 2 M1. (a) greater rate of Tests For Inorganic Ions This experiment involves identifying the cations and anions in various salt solutions. Includes kit list and safety instructions. Learn how to separate and analyse chemicals using filtration; This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Instrumental methods of analysis are faster, and more. Chemists often. Tests For Inorganic Ions.
From chemlab.truman.edu
Quantitative Analysis Chem Lab Tests For Inorganic Ions Then, test yourself with our. Students note their own observations and use their knowledge to discover more. Learn how to separate and analyse chemicals using filtration; Flame tests and chemical tests are used to detect and identify ions in samples. Instrumental methods of analysis are faster, and more. This experiment involves identifying the cations and anions in various salt solutions.. Tests For Inorganic Ions.
From www.youtube.com
GCSE 19 Separate Chemistry 9 Core Practical Testing for Ions YouTube Tests For Inorganic Ions Students will meet chemical tests that. This experiment involves identifying the cations and anions in various salt solutions. Chemists often have to identify the composition of unknown substances. Learn how to separate and analyse chemicals using filtration; Flame tests and chemical tests are used to detect and identify ions in samples. A definitive and unique response to a particular substance. Tests For Inorganic Ions.
From chem.libretexts.org
6 Qualitative Analysis of Group I Ions (Experiment) Chemistry LibreTexts Tests For Inorganic Ions Chemists often have to identify the composition of unknown substances. A definitive and unique response to a particular substance (ion, molecule or atom). A good chemical test needs to give: This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Students will meet chemical tests that. Flame. Tests For Inorganic Ions.
From www.showme.com
Topic Ions ShowMe Online Learning Tests For Inorganic Ions This experiment involves identifying the cations and anions in various salt solutions. Learn how to separate and analyse chemicals using filtration; Students will meet chemical tests that. Chemists often have to identify the composition of unknown substances. Guide students into testing for negative ions with this practical. Flame tests and chemical tests are used to detect and identify ions in. Tests For Inorganic Ions.
From www.savemyexams.com
Ions OCR A Level Biology Revision Notes 2017 Tests For Inorganic Ions Includes kit list and safety instructions. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Then, test yourself with our. This experiment involves identifying the cations and anions in various salt solutions. Students note their own observations and use their knowledge to discover more. Test for group 2 ions: Flame tests and. Tests For Inorganic Ions.
From www.tes.com
BioA_3.1.8 Ions Teaching Resources Tests For Inorganic Ions Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Includes kit list and safety instructions. A good chemical test needs to give: Students will meet chemical tests that. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Chemists. Tests For Inorganic Ions.
From www.studocu.com
AQA Chemistry All Chemical Tests Table AQA A Level Chemistry ALL Tests For Inorganic Ions Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Includes kit list and safety instructions. Then, test yourself with our. A good chemical test needs to give: Test for group 2 ions: This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed. Tests For Inorganic Ions.
From chemistry.com.pk
Testing for Cations By Sodium Hydroxide & Ammonia Precipitates Tests For Inorganic Ions Guide students into testing for negative ions with this practical. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. A good chemical test needs to give: Instrumental methods of analysis are faster, and more. Use our note to revise the tests for group 2, ammonium, halide,. Tests For Inorganic Ions.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Tests For Inorganic Ions Then, test yourself with our. Guide students into testing for negative ions with this practical. Chemists often have to identify the composition of unknown substances. Test for group 2 ions: Students note their own observations and use their knowledge to discover more. Includes kit list and safety instructions. This experiment involves identifying the cations and anions in various salt solutions.. Tests For Inorganic Ions.
From www.youtube.com
Qualitative Analysis How to distinguish lead(II) ions from aluminium Tests For Inorganic Ions Then, test yourself with our. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. This experiment involves identifying the cations and anions in various salt solutions. Learn how to separate and analyse chemicals using filtration; Test for group 2 ions: This page describes and explains the tests for halide ions (fluoride, chloride,. Tests For Inorganic Ions.
From www.youtube.com
TEST aqueous solutions for identifying IONS. Chemistry YouTube Tests For Inorganic Ions Flame tests and chemical tests are used to detect and identify ions in samples. This experiment involves identifying the cations and anions in various salt solutions. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Includes kit list and safety instructions. This page describes and explains the tests for halide ions (fluoride,. Tests For Inorganic Ions.
From zhtutorials.com
Ions Biological Molecules Ep 10 Zoë Huggett Tutorials Tests For Inorganic Ions Guide students into testing for negative ions with this practical. Test for group 2 ions: Flame tests and chemical tests are used to detect and identify ions in samples. Instrumental methods of analysis are faster, and more. Includes kit list and safety instructions. Chemists often have to identify the composition of unknown substances. This experiment involves identifying the cations and. Tests For Inorganic Ions.
From gmonbac.com
TP Constitution du Nigari gmonbac Tests For Inorganic Ions Students will meet chemical tests that. Includes kit list and safety instructions. Instrumental methods of analysis are faster, and more. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. This experiment involves identifying the cations and anions in various salt solutions. A definitive and unique response. Tests For Inorganic Ions.
From thescienceteacher.co.uk
Tests for ions and gases teaching resources the science teacher Tests For Inorganic Ions A definitive and unique response to a particular substance (ion, molecule or atom). Guide students into testing for negative ions with this practical. Flame tests and chemical tests are used to detect and identify ions in samples. Test for group 2 ions: Chemists often have to identify the composition of unknown substances. This page describes and explains the tests for. Tests For Inorganic Ions.
From www.tes.com
OnSpecScience Teaching Resources TES Tests For Inorganic Ions Students will meet chemical tests that. Learn how to separate and analyse chemicals using filtration; A good chemical test needs to give: Guide students into testing for negative ions with this practical. A definitive and unique response to a particular substance (ion, molecule or atom). Instrumental methods of analysis are faster, and more. This experiment involves identifying the cations and. Tests For Inorganic Ions.
From dxonmwhdf.blob.core.windows.net
Quantitative Analysis Laboratory Chemistry at Kathleen Reyes blog Tests For Inorganic Ions Flame tests and chemical tests are used to detect and identify ions in samples. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Students note their own observations and use their knowledge to discover more. A definitive and unique response to a particular substance (ion, molecule or atom). Instrumental methods of analysis. Tests For Inorganic Ions.
From sciencesphysiques.e-monsite.com
Test des ions Tests For Inorganic Ions This experiment involves identifying the cations and anions in various salt solutions. Students will meet chemical tests that. Guide students into testing for negative ions with this practical. Instrumental methods of analysis are faster, and more. A definitive and unique response to a particular substance (ion, molecule or atom). Learn how to separate and analyse chemicals using filtration; Test for. Tests For Inorganic Ions.
From www.youtube.com
A Level Biology ions" YouTube Tests For Inorganic Ions Instrumental methods of analysis are faster, and more. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. Learn how to separate and analyse chemicals using filtration; Guide students into testing for negative ions with this practical. A good chemical test needs to give: Students will meet. Tests For Inorganic Ions.
From www.tes.com
Ions Alevel Biology AQA Teaching Resources Tests For Inorganic Ions Test for group 2 ions: Then, test yourself with our. A good chemical test needs to give: Students will meet chemical tests that. Instrumental methods of analysis are faster, and more. Chemists often have to identify the composition of unknown substances. This experiment involves identifying the cations and anions in various salt solutions. This page describes and explains the tests. Tests For Inorganic Ions.
From www.youtube.com
EVERY Aqueous Ion OBSERVATION and EQUATION You NEED To Know|A Tests For Inorganic Ions Instrumental methods of analysis are faster, and more. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. A good chemical test needs to give: A definitive and unique response. Tests For Inorganic Ions.
From atleticofondeadero.blogspot.com
Ion Color Chart Chemistry 19 2 Coordination Chemistry Of Transition Tests For Inorganic Ions Learn how to separate and analyse chemicals using filtration; Then, test yourself with our. Guide students into testing for negative ions with this practical. Flame tests and chemical tests are used to detect and identify ions in samples. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Includes kit list and safety. Tests For Inorganic Ions.
From www.youtube.com
ions AQA A Level Biology YouTube Tests For Inorganic Ions Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Students note their own observations and use their knowledge to discover more. Learn how to separate and analyse chemicals using filtration; A good chemical test needs to give: Instrumental methods of analysis are faster, and more. Test for group 2 ions: This experiment. Tests For Inorganic Ions.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Tests For Inorganic Ions Students will meet chemical tests that. This page describes and explains the tests for halide ions (fluoride, chloride, bromide and iodide) using silver nitrate solution followed by ammonia solution. A definitive and unique response to a particular substance (ion, molecule or atom). Guide students into testing for negative ions with this practical. Learn how to separate and analyse chemicals using. Tests For Inorganic Ions.
From www.youtube.com
GCSE CHEMISTRY CHEMICAL TESTS LESSON 2 test for cations aqueous Tests For Inorganic Ions Chemists often have to identify the composition of unknown substances. Guide students into testing for negative ions with this practical. A good chemical test needs to give: A definitive and unique response to a particular substance (ion, molecule or atom). Students will meet chemical tests that. Flame tests and chemical tests are used to detect and identify ions in samples.. Tests For Inorganic Ions.
From chem.libretexts.org
3.6 Names and Formulas of Compounds Chemistry LibreTexts Tests For Inorganic Ions Chemists often have to identify the composition of unknown substances. A good chemical test needs to give: Flame tests and chemical tests are used to detect and identify ions in samples. This experiment involves identifying the cations and anions in various salt solutions. Students will meet chemical tests that. This page describes and explains the tests for halide ions (fluoride,. Tests For Inorganic Ions.
From www.thestudentroom.co.uk
AQA chem module 5...transition metal colours and ions etc Tests For Inorganic Ions A definitive and unique response to a particular substance (ion, molecule or atom). Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Then, test yourself with our. Instrumental methods of analysis are faster, and more. A good chemical test needs to give: Students will meet chemical tests that. Flame tests and chemical. Tests For Inorganic Ions.
From chemistry.com.pk
Colours of Transition Metal Ions in Aqueous Solution [Infographic Tests For Inorganic Ions Then, test yourself with our. Guide students into testing for negative ions with this practical. Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. This experiment involves identifying the cations and anions in various salt solutions. Students will meet chemical tests that. A good chemical test needs to give: Students note their. Tests For Inorganic Ions.
From www.worksheeto.com
15 Best Images of Practice Naming Ionic Compounds Worksheet Naming Tests For Inorganic Ions Then, test yourself with our. A definitive and unique response to a particular substance (ion, molecule or atom). Use our note to revise the tests for group 2, ammonium, halide, hydroxide, sulfate and carbonate ions. Test for group 2 ions: Learn how to separate and analyse chemicals using filtration; Chemists often have to identify the composition of unknown substances. This. Tests For Inorganic Ions.
From www.linstitute.net
AQA A Level Chemistry复习笔记4.2.2 Identifying Anions & Cations翰林国际教育 Tests For Inorganic Ions Then, test yourself with our. Guide students into testing for negative ions with this practical. Students note their own observations and use their knowledge to discover more. This experiment involves identifying the cations and anions in various salt solutions. Includes kit list and safety instructions. Chemists often have to identify the composition of unknown substances. Test for group 2 ions:. Tests For Inorganic Ions.
From www.youtube.com
Testing for Halide Ions, Experiment and Explanations. ALevel Chemistry Tests For Inorganic Ions Then, test yourself with our. Includes kit list and safety instructions. Instrumental methods of analysis are faster, and more. Students note their own observations and use their knowledge to discover more. A definitive and unique response to a particular substance (ion, molecule or atom). Guide students into testing for negative ions with this practical. This page describes and explains the. Tests For Inorganic Ions.