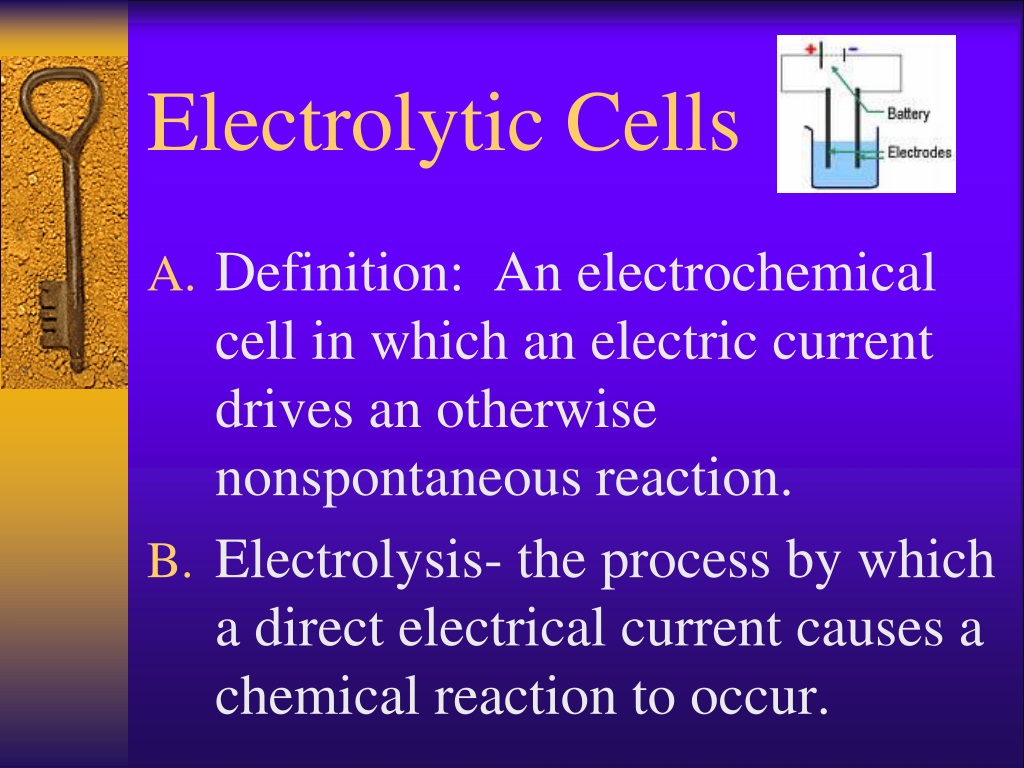
Electrolytic Cell Problems . for each of the following reactions below, draw a voltaic cell. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolytic cells problems and solutions. We will also learn broadly about. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). In your drawing include the anode, cathode, and show the flow of electrons. Here, the redox reaction is spontaneous and is. these cells are called electrolytic cells. In this tutorial, we will learn about a second type of electrochemical cell: an electrolytic cell converts electrical energy into chemical energy. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative.
from www.slideserve.com
In this tutorial, we will learn about a second type of electrochemical cell: In your drawing include the anode, cathode, and show the flow of electrons. an electrolytic cell converts electrical energy into chemical energy. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. electrolytic cells problems and solutions. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). these cells are called electrolytic cells. for each of the following reactions below, draw a voltaic cell. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Here, the redox reaction is spontaneous and is.
PPT Electrochemistry PowerPoint Presentation, free download ID9399604
Electrolytic Cell Problems an electrolytic cell converts electrical energy into chemical energy. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. We will also learn broadly about. In this tutorial, we will learn about a second type of electrochemical cell: electrolytic cells problems and solutions. In your drawing include the anode, cathode, and show the flow of electrons. Here, the redox reaction is spontaneous and is. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. for each of the following reactions below, draw a voltaic cell. an electrolytic cell converts electrical energy into chemical energy. these cells are called electrolytic cells.
From www.youtube.com
Cell Potential Problems Electrochemistry YouTube Electrolytic Cell Problems electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. In this tutorial, we will learn about a second type of electrochemical cell: Here, the redox reaction is spontaneous and is. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). an electrolytic cell converts electrical energy into chemical. Electrolytic Cell Problems.
From www.youtube.com
Calculating Cell Potential and Electrolytic Cell Problems YouTube Electrolytic Cell Problems In this tutorial, we will learn about a second type of electrochemical cell: Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolytic cells problems and solutions. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and. Electrolytic Cell Problems.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic/Galvanic Cell Electrolytic Cell Problems What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). In your drawing include the anode, cathode, and show the flow of electrons. an electrolytic cell converts electrical energy into chemical energy. electrolysis is a fundamental part of chemistry, which involves breaking. Electrolytic Cell Problems.
From slidetodoc.com
Electrolytic Cells Lab Lesson 10 Electrolytic cell Lab Electrolytic Cell Problems electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. an electrolytic cell converts electrical energy into chemical energy. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). Here, the redox reaction. Electrolytic Cell Problems.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 JEE IIT) YouTube Electrolytic Cell Problems Here, the redox reaction is spontaneous and is. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. electrolytic cells problems and solutions. In your drawing include the anode, cathode, and show the flow of electrons. an electrolytic cell converts electrical energy into chemical energy. Balance the equation. Electrolytic Cell Problems.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic... Download Scientific Diagram Electrolytic Cell Problems electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). these cells are called electrolytic cells. electrolytic cells problems and solutions. an electrolytic cell converts electrical energy into chemical energy. for each of the following reactions. Electrolytic Cell Problems.
From www.dreamstime.com
Electrolytic Cell Infographic Diagram with Components Stock Vector Illustration of education Electrolytic Cell Problems Here, the redox reaction is spontaneous and is. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. these cells are called electrolytic cells. for each of the following reactions below, draw a voltaic cell. In this tutorial, we will learn about a second type of electrochemical cell:. Electrolytic Cell Problems.
From www.youtube.com
electrolytic cells practice problems YouTube Electrolytic Cell Problems for each of the following reactions below, draw a voltaic cell. In your drawing include the anode, cathode, and show the flow of electrons. Here, the redox reaction is spontaneous and is. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. What volume of o 2 (g) at. Electrolytic Cell Problems.
From www.youtube.com
Emf of daniel cell from nernst equation(Electrochemistry part 28 for CBSE class 12 JEE IIT Electrolytic Cell Problems What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. an electrolytic cell converts electrical energy into chemical energy. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). Here, the redox reaction is spontaneous and is. these cells are called electrolytic cells. electrolytic cells problems and solutions. In your drawing. Electrolytic Cell Problems.
From studylib.net
Electrochemical cells Electrolytic Cell Problems Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. these cells are called electrolytic cells. In your drawing include the anode, cathode, and show the flow of electrons. an electrolytic cell converts electrical energy into chemical energy.. Electrolytic Cell Problems.
From thechemistrynotes.com
Electrolytic Cell Definition, Principle, Components, Applications Electrolytic Cell Problems Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). In this tutorial, we will learn about a second type of electrochemical cell: What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolytic cells problems and solutions. an electrolytic cell converts electrical energy into chemical energy. these cells are called. Electrolytic Cell Problems.
From www.youtube.com
Cell Notation Practice Problems, Voltaic Cells Electrochemistry YouTube Electrolytic Cell Problems In this tutorial, we will learn about a second type of electrochemical cell: electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. We will also learn broadly about. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). In your drawing include the anode, cathode, and show the flow. Electrolytic Cell Problems.
From www.numerade.com
SOLVED Use line notation to represent each electrochemical cell in Problem 43 . Numerade Electrolytic Cell Problems In this tutorial, we will learn about a second type of electrochemical cell: electrolytic cells problems and solutions. for each of the following reactions below, draw a voltaic cell. We will also learn broadly about. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Here, the redox reaction is spontaneous and. Electrolytic Cell Problems.
From school.careers360.com
Electrochemical Cell Overview, Structure, Properties & Uses Electrolytic Cell Problems these cells are called electrolytic cells. We will also learn broadly about. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. In this tutorial, we will learn about a second type of electrochemical cell: electrolytic cells problems and solutions. an electrolytic cell converts electrical energy into chemical energy. electrolysis. Electrolytic Cell Problems.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 Electrolytic Cell Problems We will also learn broadly about. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. In this tutorial, we will learn about a second type of electrochemical cell: Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). an electrolytic cell converts electrical energy into chemical energy. . Electrolytic Cell Problems.
From studylib.net
practice problems electrochemical cell Electrolytic Cell Problems these cells are called electrolytic cells. electrolytic cells problems and solutions. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. In. Electrolytic Cell Problems.
From www.aapp.in
Electrochemistry Introduction, Principle, Conductivity, Factors affecting electrolytic Electrolytic Cell Problems an electrolytic cell converts electrical energy into chemical energy. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. these cells are. Electrolytic Cell Problems.
From www.youtube.com
Chapter 8 electrochemical cells Problems YouTube Electrolytic Cell Problems In your drawing include the anode, cathode, and show the flow of electrons. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolytic cells problems and solutions. these cells are called electrolytic cells. Here, the redox reaction is spontaneous and is.. Electrolytic Cell Problems.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download ID2209364 Electrolytic Cell Problems Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). these cells are called electrolytic cells. Here, the redox reaction is spontaneous and is. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolytic cells problems and solutions. for each of the following reactions below, draw a voltaic cell. We. Electrolytic Cell Problems.
From byjus.com
Flow of electrons and current in electrolytic cell in internal and external supply. Electrolytic Cell Problems What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolytic cells problems and solutions. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). for each of the following reactions below, draw a voltaic cell. an electrolytic cell converts electrical energy into chemical energy. In this tutorial, we will learn. Electrolytic Cell Problems.
From facts.net
16 Mindblowing Facts About Electrolytic Cell Electrolytic Cell Problems for each of the following reactions below, draw a voltaic cell. We will also learn broadly about. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. Here, the redox reaction is spontaneous and is. In this tutorial, we will learn about a second type of electrochemical cell: Balance. Electrolytic Cell Problems.
From www.researchgate.net
Schematic diagram of electrolytic cell Download Scientific Diagram Electrolytic Cell Problems these cells are called electrolytic cells. We will also learn broadly about. Here, the redox reaction is spontaneous and is. In this tutorial, we will learn about a second type of electrochemical cell: In your drawing include the anode, cathode, and show the flow of electrons. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). What volume of. Electrolytic Cell Problems.
From www.reddit.com
Electrolytic cell electron movement r/Mcat Electrolytic Cell Problems What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. We will also learn broadly about. for each of the following reactions below, draw a voltaic cell. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation. Electrolytic Cell Problems.
From www.youtube.com
Electrolytic Cells Problem Solving Tips V03 YouTube Electrolytic Cell Problems electrolytic cells problems and solutions. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. for each of the following reactions below, draw a voltaic cell. In this tutorial, we will learn about a second type of electrochemical cell: Balance the equation and calculate the \ (\mathrm {e^\circ_. Electrolytic Cell Problems.
From www.researchgate.net
(a) Schematic diagram of an electrolytic cell (Reprinted with... Download Scientific Diagram Electrolytic Cell Problems electrolytic cells problems and solutions. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. an electrolytic cell converts electrical energy into chemical energy. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. for each of the following reactions below,. Electrolytic Cell Problems.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Electrolytic Cell Problems What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Here, the redox reaction is spontaneous and is. In this tutorial, we will learn about a second type of electrochemical cell: an electrolytic cell converts electrical energy into chemical energy. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). for each. Electrolytic Cell Problems.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID9399604 Electrolytic Cell Problems What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. an electrolytic cell converts electrical energy into chemical energy. electrolytic cells problems and solutions. these cells are called electrolytic cells. In. Electrolytic Cell Problems.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5570772 Electrolytic Cell Problems In your drawing include the anode, cathode, and show the flow of electrons. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). electrolytic cells problems and solutions. In this tutorial, we will learn about a second type of electrochemical cell: these cells are called electrolytic cells. for each of the following reactions below, draw a voltaic. Electrolytic Cell Problems.
From wisc.pb.unizin.org
M18Q8 Electrolysis Chem 103/104 Resource Book Electrolytic Cell Problems In this tutorial, we will learn about a second type of electrochemical cell: We will also learn broadly about. Here, the redox reaction is spontaneous and is. electrolytic cells problems and solutions. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. these cells are called electrolytic cells.. Electrolytic Cell Problems.
From www.slideserve.com
PPT The Electrolytic Cell PowerPoint Presentation, free download ID6584924 Electrolytic Cell Problems Here, the redox reaction is spontaneous and is. an electrolytic cell converts electrical energy into chemical energy. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. electrolysis is a fundamental part of chemistry, which involves breaking of electrolyte(the solution) and formation of positive and negative. electrolytic cells problems and solutions.. Electrolytic Cell Problems.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrolytic Cell Problems We will also learn broadly about. electrolytic cells problems and solutions. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). In this tutorial, we will learn about a second type of electrochemical cell: an electrolytic cell converts electrical energy into chemical. Electrolytic Cell Problems.
From www.studocu.com
Electrolytic cells questions ELECTROLYTIC CELLS QUESTION 1 The simplified diagrams below Electrolytic Cell Problems Here, the redox reaction is spontaneous and is. Balance the equation and calculate the \ (\mathrm {e^\circ_ {cell}}\). these cells are called electrolytic cells. In your drawing include the anode, cathode, and show the flow of electrons. an electrolytic cell converts electrical energy into chemical energy. electrolysis is a fundamental part of chemistry, which involves breaking of. Electrolytic Cell Problems.
From www.youtube.com
19.Electrolytic Cell & Electrolysis Problems Electrochemistry Class 12 NCERT in Tamil Electrolytic Cell Problems these cells are called electrolytic cells. an electrolytic cell converts electrical energy into chemical energy. We will also learn broadly about. for each of the following reactions below, draw a voltaic cell. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. Balance the equation and calculate the \ (\mathrm {e^\circ_. Electrolytic Cell Problems.
From www.youtube.com
What is Electrolytic cell in Simple Words Electrolytic Cell Animation Electrochemistry YouTube Electrolytic Cell Problems for each of the following reactions below, draw a voltaic cell. electrolytic cells problems and solutions. We will also learn broadly about. What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. these cells are called electrolytic cells. In your drawing include the anode, cathode, and show the flow of electrons.. Electrolytic Cell Problems.
From employees.csbsju.edu
CHEM 123 GENERAL CHEMISTRY 1 Electrolytic Cell Problems Here, the redox reaction is spontaneous and is. these cells are called electrolytic cells. In this tutorial, we will learn about a second type of electrochemical cell: What volume of o 2 (g) at 0 o c and 760 mmhg is produced when. for each of the following reactions below, draw a voltaic cell. an electrolytic cell. Electrolytic Cell Problems.