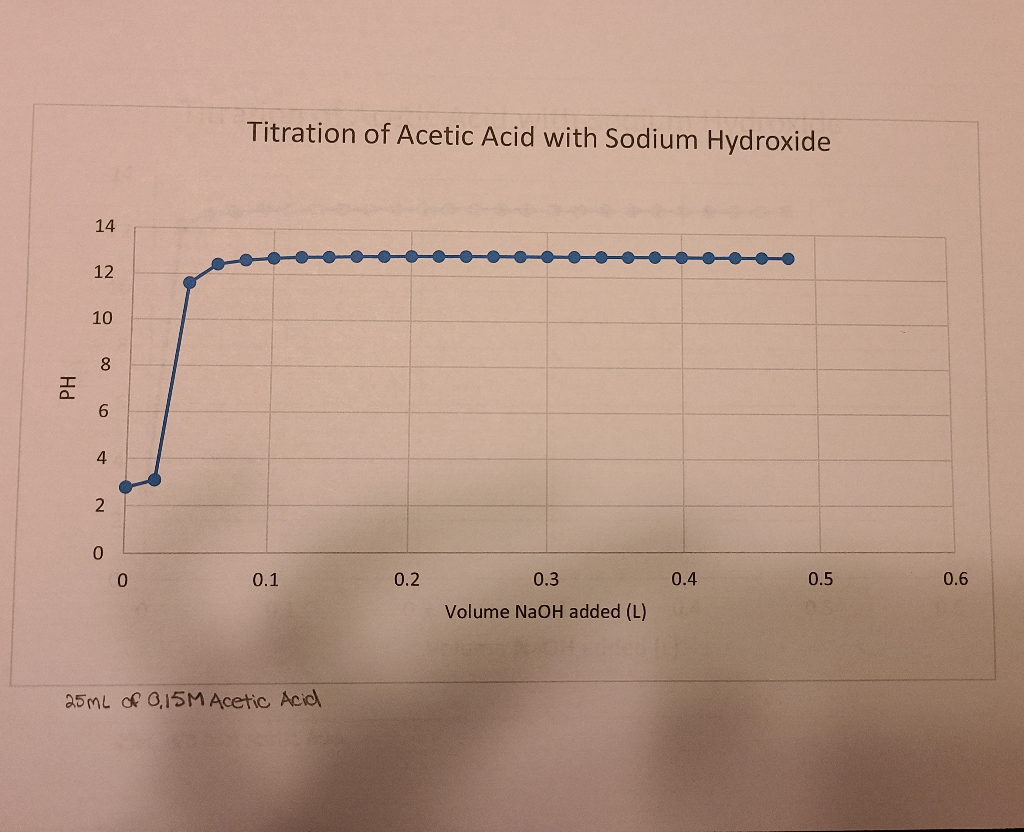
Titration Calculations Acetic Acid And Sodium Hydroxide . in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A) what is the ph of the solution before you add any of the naoh? — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. You start off with 50ml of a.3 m acetic acid solution. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. B) suppose you have 50 ml of a.3 m solution of acetic acid. Initially the ph is due to pure acetic acid.
from mavink.com
titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. A) what is the ph of the solution before you add any of the naoh? Initially the ph is due to pure acetic acid. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: B) suppose you have 50 ml of a.3 m solution of acetic acid. You start off with 50ml of a.3 m acetic acid solution. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar).
Sodium Hydroxide Ph Chart
Titration Calculations Acetic Acid And Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. You start off with 50ml of a.3 m acetic acid solution. Initially the ph is due to pure acetic acid. A) what is the ph of the solution before you add any of the naoh? the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. B) suppose you have 50 ml of a.3 m solution of acetic acid.
From www.tessshebaylo.com
Write The Balanced Equation Showing Ionization Of Formic Acid In Water Tessshebaylo Titration Calculations Acetic Acid And Sodium Hydroxide Initially the ph is due to pure acetic acid. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: B) suppose you have 50 ml of a.3 m solution of acetic acid. when a weak acid such as acetic acid is titrated with a strong. Titration Calculations Acetic Acid And Sodium Hydroxide.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Calculations Acetic Acid And Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). A) what is the ph of the solution before you add any of the naoh? when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. Initially the. Titration Calculations Acetic Acid And Sodium Hydroxide.
From childhealthpolicy.vumc.org
⭐ Acetic acid and naoh titration. Titration of NaOH with acetic acid. 20221022 Titration Calculations Acetic Acid And Sodium Hydroxide A) what is the ph of the solution before you add any of the naoh? the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when a weak acid such as acetic acid is titrated with a strong base. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Titration Calculations Acetic Acid And Sodium Hydroxide in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A) what is the ph of the solution before you add any of the naoh? B) suppose you have 50 ml of a.3 m solution of acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. when. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.researchgate.net
Titration curve of acetic acid at 30 °C (Ve = equivalence volume) Download Scientific Diagram Titration Calculations Acetic Acid And Sodium Hydroxide in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. A) what is the ph of the solution before you add any of the naoh? when a weak acid such as acetic acid is titrated with a strong base. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.tutormyself.com
233 Archives TutorMyself Chemistry Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. A) what is the ph of the solution before you add any of the naoh? — in this lab, you will perform a titration using sodium hydroxide and. Titration Calculations Acetic Acid And Sodium Hydroxide.
From mavink.com
Sodium Hydroxide Ph Chart Titration Calculations Acetic Acid And Sodium Hydroxide in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. A) what is the ph of the solution before you add any of the naoh? the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. when a. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.chegg.com
Solved 1. (a) A titration of 5.00 mL acetic acid required Titration Calculations Acetic Acid And Sodium Hydroxide when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. Initially the ph is due to pure acetic acid. You start off with 50ml of a.3 m acetic acid solution. — in this lab, you will perform a titration using sodium hydroxide and acetic. Titration Calculations Acetic Acid And Sodium Hydroxide.
From exoqledql.blob.core.windows.net
Titration Base Hydrochloric Acid at Bryan Barnes blog Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. A) what is the ph of the solution before you add any of the naoh? in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: You start off with 50ml of a.3 m acetic acid solution. Initially the ph is due to pure acetic acid.. Titration Calculations Acetic Acid And Sodium Hydroxide.
From chart-studio.plotly.com
Acetic Acid (CH3COOH) + Sodium Hydroxide (NaOH) Titration scatter chart made by Crawfordc1 Titration Calculations Acetic Acid And Sodium Hydroxide A) what is the ph of the solution before you add any of the naoh? B) suppose you have 50 ml of a.3 m solution of acetic acid. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. You start off with 50ml of a.3. Titration Calculations Acetic Acid And Sodium Hydroxide.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Calculations Acetic Acid And Sodium Hydroxide You start off with 50ml of a.3 m acetic acid solution. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. — in this lab, you will perform a titration using sodium. Titration Calculations Acetic Acid And Sodium Hydroxide.
From sciencestruck.com
Titration of Sulfuric Acid and Sodium Hydroxide Titration Calculations Acetic Acid And Sodium Hydroxide when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: B) suppose you have 50 ml of a.3 m solution of. Titration Calculations Acetic Acid And Sodium Hydroxide.
From chempedia.info
Sodium hydroxide titration with acetic acid Big Chemical Encyclopedia Titration Calculations Acetic Acid And Sodium Hydroxide You start off with 50ml of a.3 m acetic acid solution. Initially the ph is due to pure acetic acid. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when a weak acid such as acetic acid is. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED 1. Which indicator is used to determine the endpoint of the reaction between acetic acid Titration Calculations Acetic Acid And Sodium Hydroxide when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: Initially the ph is due. Titration Calculations Acetic Acid And Sodium Hydroxide.
From feqtudy.blogspot.com
Indicator For Titration Of Acetic Acid And Sodium Hydroxide FEQTUDY Titration Calculations Acetic Acid And Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. B) suppose you have 50 ml of a.3 m solution of acetic acid. You start off with 50ml of a.3 m acetic acid solution.. Titration Calculations Acetic Acid And Sodium Hydroxide.
From mungfali.com
Titration Curve Of Acetic Acid Titration Calculations Acetic Acid And Sodium Hydroxide A) what is the ph of the solution before you add any of the naoh? in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). when a. Titration Calculations Acetic Acid And Sodium Hydroxide.
From exosjwdrb.blob.core.windows.net
Titration Calculation Table Method at Susan Clyburn blog Titration Calculations Acetic Acid And Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. You start off with 50ml. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED Experiment 10 Acid Dissociation Constant by Titration 0952 Concentration standard NaOH Titration Calculations Acetic Acid And Sodium Hydroxide the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. Initially the ph is due to pure acetic acid. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. You start off with 50ml of a.3 m. Titration Calculations Acetic Acid And Sodium Hydroxide.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. You start off with 50ml of a.3 m acetic acid solution. titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: A) what is the ph of the solution before you add any. Titration Calculations Acetic Acid And Sodium Hydroxide.
From snipe.fm
😎 Acetic acid and naoh titration. Weak Acid. 20190130 Titration Calculations Acetic Acid And Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: when a weak acid such as acetic acid is titrated with. Titration Calculations Acetic Acid And Sodium Hydroxide.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Calculations Acetic Acid And Sodium Hydroxide when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar. Titration Calculations Acetic Acid And Sodium Hydroxide.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators of Acid Base Titration Titration Calculations Acetic Acid And Sodium Hydroxide Initially the ph is due to pure acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. You start off with 50ml of a.3 m acetic acid solution. A) what is the ph of the solution before you add any of the naoh? in a titration of sulfuric acid. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. Initially the ph is due to pure acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. A) what is the ph of the solution before you add any of the naoh? You start off with 50ml. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.studypool.com
SOLUTION Chemistry experiment conductometric titration of hydrochloric acid and acetic acid Titration Calculations Acetic Acid And Sodium Hydroxide when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. You start off with 50ml of a.3 m acetic acid solution. A) what is the ph of the solution before you add any of the naoh? — in this lab, you will perform a. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.tessshebaylo.com
Write The Balanced Net Ionic Equation For Dissociation Of Acetic Acid In Water Tessshebaylo Titration Calculations Acetic Acid And Sodium Hydroxide the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: You start off with 50ml of a.3 m acetic acid solution. Initially the ph is due to pure acetic acid. titration of acetic acid with sodium hydroxide (analagous to. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.vrogue.co
Acid Base Titration Calculation Molar Concentration T vrogue.co Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. Initially the ph is due to pure acetic acid. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. the goal of this experiment is to determine accurately the concentration of. Titration Calculations Acetic Acid And Sodium Hydroxide.
From mavink.com
Titration Curve Of Acetic Acid Titration Calculations Acetic Acid And Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. A) what is the ph of the solution before you add any of the naoh? You start off with 50ml of a.3 m. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED Calculate the pH in the titration of 25.0 mL of 0.100 M ethanoic acid (Ka= 1.8 x 105 Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. Initially the ph is due to pure acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. You start off with 50ml of a.3 m acetic acid solution. — in this lab, you will perform a. Titration Calculations Acetic Acid And Sodium Hydroxide.
From chempedia.info
Sodium hydroxide titration with acetic acid Big Chemical Encyclopedia Titration Calculations Acetic Acid And Sodium Hydroxide A) what is the ph of the solution before you add any of the naoh? B) suppose you have 50 ml of a.3 m solution of acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. You start off with 50ml of a.3 m acetic acid solution. — in. Titration Calculations Acetic Acid And Sodium Hydroxide.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Titration Calculations Acetic Acid And Sodium Hydroxide in a titration of sulfuric acid against sodium hydroxide, \(32.20 \: — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). B) suppose you have 50 ml of a.3 m solution of acetic acid. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar. Titration Calculations Acetic Acid And Sodium Hydroxide.
From fr.slideserve.com
PPT Common Types of Reactions PowerPoint Presentation, free download ID4565120 Titration Calculations Acetic Acid And Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. You start off with 50ml of a.3 m acetic acid solution. Initially the ph is due to pure acetic acid. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). in a titration of sulfuric acid against sodium hydroxide,. Titration Calculations Acetic Acid And Sodium Hydroxide.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Titration Calculations Acetic Acid And Sodium Hydroxide — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). A) what is the ph of the solution before you add any of the naoh? titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. the goal of this experiment is to determine accurately the concentration of acetic acid. Titration Calculations Acetic Acid And Sodium Hydroxide.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Calculations Acetic Acid And Sodium Hydroxide B) suppose you have 50 ml of a.3 m solution of acetic acid. when a weak acid such as acetic acid is titrated with a strong base such as aqueous sodium hydroxide solution, the ph at the. A) what is the ph of the solution before you add any of the naoh? titration of acetic acid with sodium. Titration Calculations Acetic Acid And Sodium Hydroxide.
From www.numerade.com
SOLVED The following data is from a conductometric titration of 10.00 mL aqueous solution Titration Calculations Acetic Acid And Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. B) suppose you have 50 ml of a.3 m solution of acetic acid. Initially the ph is due to pure acetic acid. — in this lab, you will perform a titration using sodium hydroxide and acetic acid (in vinegar). in a titration of sulfuric acid against. Titration Calculations Acetic Acid And Sodium Hydroxide.
From ayaanmcyduarte.blogspot.com
Ethanoic Acid and Sodium Hydroxide AyaanmcyDuarte Titration Calculations Acetic Acid And Sodium Hydroxide titration of acetic acid with sodium hydroxide (analagous to figure \(\pageindex{3}\)c. You start off with 50ml of a.3 m acetic acid solution. A) what is the ph of the solution before you add any of the naoh? the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. when a weak. Titration Calculations Acetic Acid And Sodium Hydroxide.