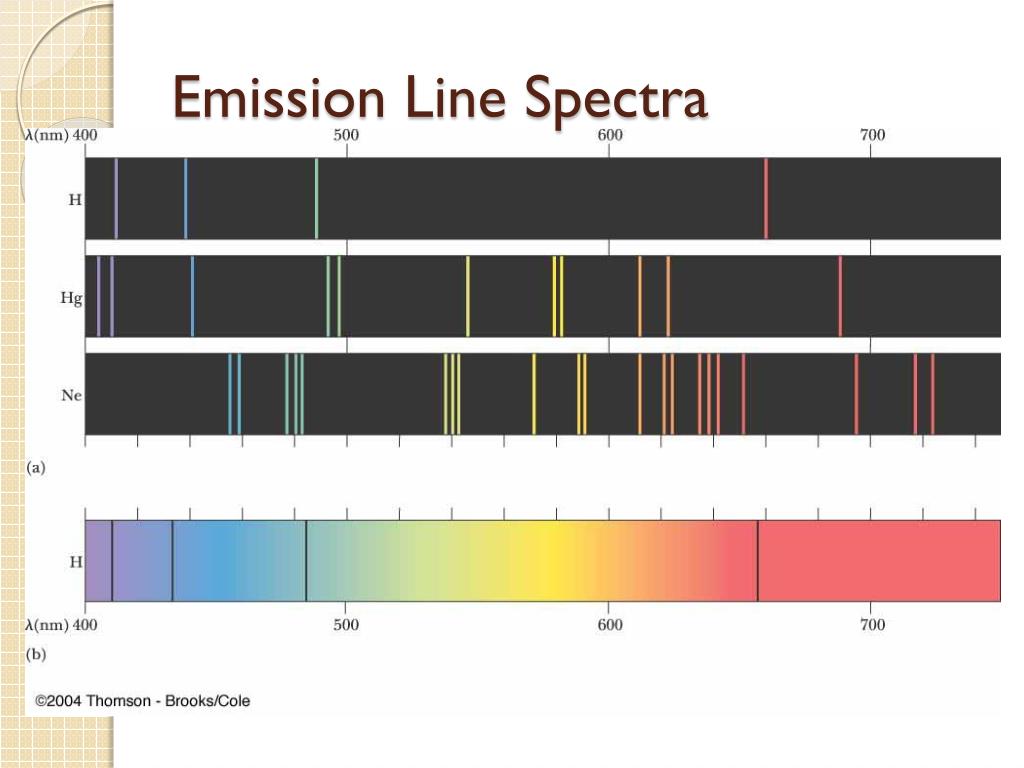
What Is Lines Emission Spectrum . You will know how spectral lines are. The atom is first excited by a colliding electron. You will be able to distinguish between emission and absorption lines in a spectrum. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. When the atom goes back to its ground state, either. there are two types of line spectra, emission and absorption. In an emission spectra electrons are excited to an excited.
from www.slideserve.com
— line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. there are two types of line spectra, emission and absorption. You will know how spectral lines are. The atom is first excited by a colliding electron. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In an emission spectra electrons are excited to an excited. When the atom goes back to its ground state, either. You will be able to distinguish between emission and absorption lines in a spectrum.
PPT Electrons PowerPoint Presentation, free download ID1995426
What Is Lines Emission Spectrum there are two types of line spectra, emission and absorption. there are two types of line spectra, emission and absorption. When the atom goes back to its ground state, either. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. In an emission spectra electrons are excited to an excited. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. You will be able to distinguish between emission and absorption lines in a spectrum. The atom is first excited by a colliding electron. You will know how spectral lines are.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room What Is Lines Emission Spectrum there are two types of line spectra, emission and absorption. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. In an emission spectra electrons are excited to an excited. You will be able to distinguish between emission and absorption lines in a spectrum. The atom is first excited. What Is Lines Emission Spectrum.
From www.astronoo.com
Spectroscopy — Astronoo What Is Lines Emission Spectrum this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. You will know how spectral lines are. When the atom goes back to its ground state, either. In an emission spectra electrons are excited to an excited. — line spectra is a phenomenon which occurs when excited atoms emit. What Is Lines Emission Spectrum.
From www.resonancescience.org
What is Resonance and Why is it so Important? What Is Lines Emission Spectrum You will know how spectral lines are. When the atom goes back to its ground state, either. The atom is first excited by a colliding electron. In an emission spectra electrons are excited to an excited. there are two types of line spectra, emission and absorption. this spectrum of radiation emitted by electrons in the excited atoms or. What Is Lines Emission Spectrum.
From winstonmcyponce.blogspot.com
Atomic Emission Spectrum of Hydrogen WinstonmcyPonce What Is Lines Emission Spectrum When the atom goes back to its ground state, either. The atom is first excited by a colliding electron. In an emission spectra electrons are excited to an excited. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. this spectrum of radiation emitted by electrons in the excited atoms. What Is Lines Emission Spectrum.
From educationgrafts.z21.web.core.windows.net
Line Emission Spectrum Of Hydrogen What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. When the atom goes back to its ground state, either. You will know how spectral lines are. The atom is first excited by a colliding electron. You will be able to distinguish between emission and absorption lines in a spectrum. . What Is Lines Emission Spectrum.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction What Is Lines Emission Spectrum You will know how spectral lines are. You will be able to distinguish between emission and absorption lines in a spectrum. In an emission spectra electrons are excited to an excited. When the atom goes back to its ground state, either. there are two types of line spectra, emission and absorption. — line spectra is a phenomenon which. What Is Lines Emission Spectrum.
From fphoto.photoshelter.com
science physics waves spectrum analysis Fundamental Photographs The Art of What Is Lines Emission Spectrum You will be able to distinguish between emission and absorption lines in a spectrum. The atom is first excited by a colliding electron. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. When the atom goes back to its ground state, either. there are two types of line spectra,. What Is Lines Emission Spectrum.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. The atom is first excited by a colliding electron. You will know how spectral lines are. You will be able to. What Is Lines Emission Spectrum.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. You will be able to distinguish between emission and absorption lines in a spectrum. The atom is first excited by a colliding electron. In an emission spectra electrons are excited to an excited. there are two types of line spectra,. What Is Lines Emission Spectrum.
From www.slideserve.com
PPT Electrons PowerPoint Presentation, free download ID1995426 What Is Lines Emission Spectrum The atom is first excited by a colliding electron. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. When the atom goes back to its ground state, either. You will know how spectral lines are. You will be able to distinguish between emission and absorption lines in a spectrum. In. What Is Lines Emission Spectrum.
From www.youtube.com
Emission spectrum of hydrogen Chemistry Khan Academy YouTube What Is Lines Emission Spectrum You will be able to distinguish between emission and absorption lines in a spectrum. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. The atom is first excited by a colliding electron. there are two types of line spectra, emission and absorption. this spectrum of radiation emitted by. What Is Lines Emission Spectrum.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum What Is Lines Emission Spectrum When the atom goes back to its ground state, either. You will know how spectral lines are. there are two types of line spectra, emission and absorption. In an emission spectra electrons are excited to an excited. The atom is first excited by a colliding electron. You will be able to distinguish between emission and absorption lines in a. What Is Lines Emission Spectrum.
From sekaangry.weebly.com
Atomic emission spectrum chemistry definition sekaangry What Is Lines Emission Spectrum You will be able to distinguish between emission and absorption lines in a spectrum. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. The atom is first excited by a colliding electron. You will know how spectral lines are. In an emission spectra electrons are excited to an excited. . What Is Lines Emission Spectrum.
From spiff.rit.edu
Spectrographs and Spectra What Is Lines Emission Spectrum The atom is first excited by a colliding electron. You will be able to distinguish between emission and absorption lines in a spectrum. You will know how spectral lines are. In an emission spectra electrons are excited to an excited. there are two types of line spectra, emission and absorption. — line spectra is a phenomenon which occurs. What Is Lines Emission Spectrum.
From infinitylearn.com
Line spectrum contains information about What Is Lines Emission Spectrum You will know how spectral lines are. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. You will be able to distinguish between emission and absorption lines in a spectrum. The atom is first excited by a colliding electron. this spectrum of radiation emitted by electrons in the excited. What Is Lines Emission Spectrum.
From jpl.nasa.gov
Educator Guide Math of the Expanding Universe NASA/JPL Edu What Is Lines Emission Spectrum You will be able to distinguish between emission and absorption lines in a spectrum. When the atom goes back to its ground state, either. You will know how spectral lines are. The atom is first excited by a colliding electron. In an emission spectra electrons are excited to an excited. there are two types of line spectra, emission and. What Is Lines Emission Spectrum.
From learningzoneteiar09.z14.web.core.windows.net
Atomic Spectra And Its Types What Is Lines Emission Spectrum In an emission spectra electrons are excited to an excited. You will know how spectral lines are. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. there are two types of line spectra, emission and absorption. When the atom goes back to its ground state, either. —. What Is Lines Emission Spectrum.
From www.dreamstime.com
The Spectrum Vector Diagram Stock Vector Illustration of nature, blue 33625774 What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. You will be able to distinguish between emission and absorption lines in a spectrum. When the atom goes back to its ground state, either. there are two types of line spectra, emission and absorption. The atom is first excited by. What Is Lines Emission Spectrum.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum. YouTube What Is Lines Emission Spectrum You will know how spectral lines are. The atom is first excited by a colliding electron. there are two types of line spectra, emission and absorption. In an emission spectra electrons are excited to an excited. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. You will be. What Is Lines Emission Spectrum.
From www.alamy.com
HHeHg emission spectra. Graphical representation of the emission spectra lines for the What Is Lines Emission Spectrum You will be able to distinguish between emission and absorption lines in a spectrum. When the atom goes back to its ground state, either. The atom is first excited by a colliding electron. You will know how spectral lines are. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. . What Is Lines Emission Spectrum.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/104 Resource Book What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. You will be able to distinguish between emission and absorption lines in a spectrum. You will know how spectral lines are. In an emission spectra electrons are excited to an excited. When the atom goes back to its ground state, either.. What Is Lines Emission Spectrum.
From terrence-kgonzales.blogspot.com
Describe the Emission Spectrum of Hydrogen Ib Chemistry What Is Lines Emission Spectrum You will be able to distinguish between emission and absorption lines in a spectrum. When the atom goes back to its ground state, either. You will know how spectral lines are. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. there are two types of line spectra, emission and. What Is Lines Emission Spectrum.
From sdsu-physics.org
Quantum Physics What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. The atom is first excited by a colliding electron. When the atom goes back to its ground state, either. You will be able to distinguish between emission and absorption lines in a spectrum. In an emission spectra electrons are excited to. What Is Lines Emission Spectrum.
From www.chemhume.co.uk
Line emission spectra What Is Lines Emission Spectrum In an emission spectra electrons are excited to an excited. The atom is first excited by a colliding electron. there are two types of line spectra, emission and absorption. When the atom goes back to its ground state, either. this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum.. What Is Lines Emission Spectrum.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra? HubPages What Is Lines Emission Spectrum When the atom goes back to its ground state, either. You will be able to distinguish between emission and absorption lines in a spectrum. there are two types of line spectra, emission and absorption. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. The atom is first excited by. What Is Lines Emission Spectrum.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts What Is Lines Emission Spectrum When the atom goes back to its ground state, either. The atom is first excited by a colliding electron. You will be able to distinguish between emission and absorption lines in a spectrum. there are two types of line spectra, emission and absorption. You will know how spectral lines are. — line spectra is a phenomenon which occurs. What Is Lines Emission Spectrum.
From byjus.com
Emission spectrum Atomic spectra Chemistry What Is Lines Emission Spectrum this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. The atom is first excited by a colliding electron. You will know how spectral lines are. You will be able to. What Is Lines Emission Spectrum.
From sixdayscience.com
Chapter 7 SixDay Science What Is Lines Emission Spectrum — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. In an emission spectra electrons are excited to an excited. When the atom goes back to its ground state, either. there are two types of line spectra, emission and absorption. this spectrum of radiation emitted by electrons in the. What Is Lines Emission Spectrum.
From socratic.org
What is line emission spectrum? + Example What Is Lines Emission Spectrum this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. there are two types of line spectra, emission and absorption. When the atom goes back to its ground state, either. In an emission spectra electrons are excited to an excited. The atom is first excited by a colliding electron.. What Is Lines Emission Spectrum.
From imagine.gsfc.nasa.gov
Spectra Introduction What Is Lines Emission Spectrum this spectrum of radiation emitted by electrons in the excited atoms or molecules is known as the emission spectrum. When the atom goes back to its ground state, either. In an emission spectra electrons are excited to an excited. You will know how spectral lines are. there are two types of line spectra, emission and absorption. —. What Is Lines Emission Spectrum.
From www.howtuo.com
The Bright Lines Of An Emission Spectrum Are The Result Of • HOWTUO What Is Lines Emission Spectrum The atom is first excited by a colliding electron. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. In an emission spectra electrons are excited to an excited. You will be able to distinguish between emission and absorption lines in a spectrum. When the atom goes back to its ground. What Is Lines Emission Spectrum.
From www.slideserve.com
PPT EMISSION SPECTRUM PowerPoint Presentation, free download ID5777174 What Is Lines Emission Spectrum In an emission spectra electrons are excited to an excited. The atom is first excited by a colliding electron. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. When the atom goes back to its ground state, either. there are two types of line spectra, emission and absorption. You. What Is Lines Emission Spectrum.
From chelsea-well-johnston.blogspot.com
Describe the Emission Spectrum of Hydrogen What Is Lines Emission Spectrum You will know how spectral lines are. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. there are two types of line spectra, emission and absorption. You will be able to distinguish between emission and absorption lines in a spectrum. The atom is first excited by a colliding electron.. What Is Lines Emission Spectrum.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog What Is Lines Emission Spectrum there are two types of line spectra, emission and absorption. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. The atom is first excited by a colliding electron. In an emission spectra electrons are excited to an excited. You will be able to distinguish between emission and absorption lines. What Is Lines Emission Spectrum.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts What Is Lines Emission Spectrum You will know how spectral lines are. The atom is first excited by a colliding electron. — line spectra is a phenomenon which occurs when excited atoms emit light of certain wavelengths which correspond. You will be able to distinguish between emission and absorption lines in a spectrum. When the atom goes back to its ground state, either. . What Is Lines Emission Spectrum.