Rectification Definition Chemistry . Rectification is a very effective separation. Rectification is an application of distillation. The article contains sections titled: Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Distillation is extensively used in chemical, petrochemical, pharmaceutical. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. It is used for substances that are required in high purity and/or large quantities, for example to. Rectification denotes a change of concentrations beyond that of simple distillation.
from www.chegg.com
Rectification denotes a change of concentrations beyond that of simple distillation. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is an application of distillation. It is used for substances that are required in high purity and/or large quantities, for example to. Rectification is a very effective separation. The article contains sections titled:
Solved 1. Define rectification. 2. What is the main function
Rectification Definition Chemistry Rectification is an application of distillation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Rectification is an application of distillation. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Rectification is a very effective separation. The article contains sections titled: Rectification denotes a change of concentrations beyond that of simple distillation.
From www.slideserve.com
PPT RECTIFICATION PowerPoint Presentation, free download ID4459485 Rectification Definition Chemistry Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is a very effective separation. The article contains sections titled: Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Rectification denotes a change of concentrations beyond that of simple distillation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points.. Rectification Definition Chemistry.
From www.chegg.com
Solved 1. Define rectification. 2. What is the main function Rectification Definition Chemistry It is used for substances that are required in high purity and/or large quantities, for example to. Rectification is a very effective separation. The article contains sections titled: Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Rectification denotes. Rectification Definition Chemistry.
From azprocede.fr
Cours de rectification Rectification Definition Chemistry Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is a very effective separation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. It is used for substances that are required in high purity and/or large quantities, for example to. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Distillation (or rectification). Rectification Definition Chemistry.
From en.ppt-online.org
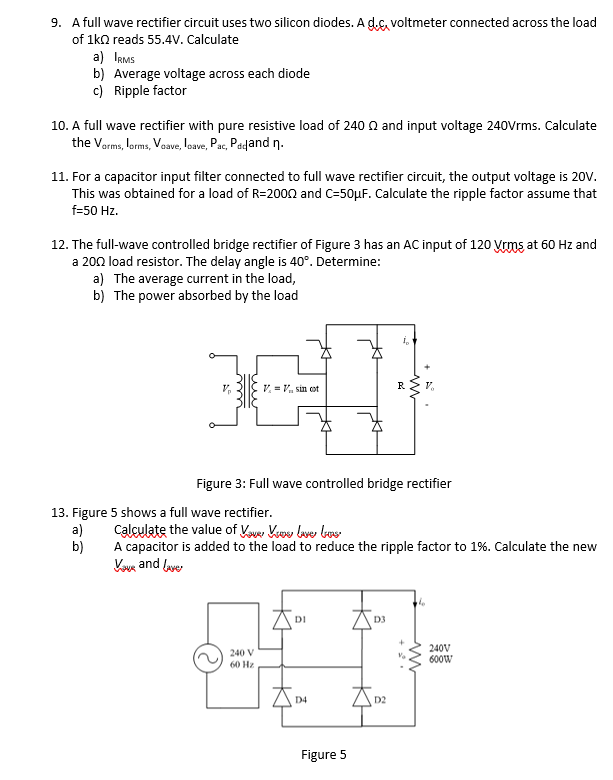
Rectification. Difference between the rectification and distillation Rectification Definition Chemistry It is used for substances that are required in high purity and/or large quantities, for example to. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is a very effective separation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. The article contains sections titled: When you rectify something, you correct an error or. Rectification Definition Chemistry.
From www.gunt.de
Rectification with CE 600 GUNT Gerätebau Rectification Definition Chemistry Rectification is an application of distillation. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Distillation is extensively used in chemical, petrochemical, pharmaceutical. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Rectification is a very effective separation. The. Rectification Definition Chemistry.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Rectification Definition Chemistry Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is an application of distillation. Rectification is a very effective separation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Fractional distillation is often used to separate mixtures of liquids that have similar. Rectification Definition Chemistry.
From askfilo.com
5. What is rectification ? Explain the working of a full wave rectifier. Rectification Definition Chemistry Rectification is a very effective separation. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is an application of distillation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. When you rectify something, you correct an error or make things right, which is. Rectification Definition Chemistry.
From study.com
Rectification Definition, Types & Purpose Lesson Rectification Definition Chemistry It is used for substances that are required in high purity and/or large quantities, for example to. The article contains sections titled: Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is a very effective separation. When you rectify something, you correct an error or. Rectification Definition Chemistry.
From www.studypool.com
SOLUTION Define and explain process of rectification Studypool Rectification Definition Chemistry Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is an application of distillation. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is a very effective separation. Fractional distillation is often used to separate mixtures of liquids that have. Rectification Definition Chemistry.
From www.slideserve.com
PPT Research Update PowerPoint Presentation, free download ID3999952 Rectification Definition Chemistry Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is an application of. Rectification Definition Chemistry.
From en.ppt-online.org
Rectification. Difference between the rectification and distillation Rectification Definition Chemistry Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is a very effective separation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. The article contains sections titled: It is used for substances that. Rectification Definition Chemistry.
From www.researchgate.net
The diagram of rectification effect in nanochannel. a The typical IV Rectification Definition Chemistry Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. When you rectify something, you correct an error or make things right, which. Rectification Definition Chemistry.
From en.ppt-online.org
Rectification. Difference between the rectification and distillation Rectification Definition Chemistry The article contains sections titled: Rectification is an application of distillation. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Rectification denotes a change of concentrations beyond that of simple distillation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back. Rectification Definition Chemistry.
From www.slideserve.com
PPT RECTIFICATION PowerPoint Presentation, free download ID4459485 Rectification Definition Chemistry Distillation is extensively used in chemical, petrochemical, pharmaceutical. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace. Rectification Definition Chemistry.
From pharmaguides.in
4.8 Rectification In Chemistry Rectification Definition Chemistry When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Rectification denotes a change of concentrations beyond that of simple distillation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Rectification is. Rectification Definition Chemistry.
From www.slchemtech.com
Difference Between Distillation and Rectification Rectification Definition Chemistry Rectification is an application of distillation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. The article contains sections titled: When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Rectification denotes. Rectification Definition Chemistry.
From www.slchemtech.com
Difference Between Distillation and Rectification Rectification Definition Chemistry The article contains sections titled: Rectification is a very effective separation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Rectification is an application of distillation. It is used for. Rectification Definition Chemistry.
From www.cell.com
Rectified Janus nanopores new vitality for ionic diodes Trends in Rectification Definition Chemistry The article contains sections titled: Rectification is an application of distillation. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. When you rectify something, you correct an error or make things right, which is fitting because rectify. Rectification Definition Chemistry.
From www.researchgate.net
Rectification ratio versus voltage changes as a function of sample Rectification Definition Chemistry The article contains sections titled: Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is an application of distillation. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. When you rectify something, you correct. Rectification Definition Chemistry.
From www.youtube.com
Rectified spirit is a mixture of CLASS 12 ALCOHOLS, PHENOLS AND Rectification Definition Chemistry Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Rectification is a very effective separation. It is used for substances that are required in high purity and/or large quantities, for. Rectification Definition Chemistry.
From www.techblade.ph
Rectification Explained Electronics for novices Rectification Definition Chemistry Rectification denotes a change of concentrations beyond that of simple distillation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. It is used for substances that are required in high. Rectification Definition Chemistry.
From en.ppt-online.org
Rectification. Difference between the rectification and distillation Rectification Definition Chemistry Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Rectification denotes a change of concentrations beyond that of simple distillation. It is used for substances that are required in high purity and/or large quantities, for example to. When you rectify something, you correct an error or make things right, which is fitting because rectify. Rectification Definition Chemistry.
From www.youtube.com
TYPES OF DISTILLATION PROCESSES FLASH, STEAM DISTILLATION AND Rectification Definition Chemistry Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Distillation is extensively used in chemical, petrochemical, pharmaceutical. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace. Rectification Definition Chemistry.
From www.researchgate.net
Schematic diagram describing the rectifying and resistive switching Rectification Definition Chemistry Rectification is an application of distillation. The article contains sections titled: Distillation is extensively used in chemical, petrochemical, pharmaceutical. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Rectification is a very effective separation. Rectification denotes a change of concentrations beyond that of simple distillation.. Rectification Definition Chemistry.
From www.simplepharmanotes.com
Fractional Distillation / Rectification. Rectification Definition Chemistry It is used for substances that are required in high purity and/or large quantities, for example to. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. The article contains sections titled: Rectification is a very effective separation. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Rectification denotes. Rectification Definition Chemistry.
From www.researchgate.net
Block diagram of the rectification procedure scheme. Download Rectification Definition Chemistry The article contains sections titled: Rectification is an application of distillation. Rectification is a very effective separation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification denotes a change of concentrations beyond that of simple distillation. Distillation (or rectification) is the most important technique for. Rectification Definition Chemistry.
From www.slideserve.com
PPT PN JUNCTION DIODE PowerPoint Presentation ID216290 Rectification Definition Chemistry Rectification denotes a change of concentrations beyond that of simple distillation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is a very effective separation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. It is used for substances that are required in high purity. Rectification Definition Chemistry.
From www.collegesearch.in
Rectifier Definition, Types, Application, Uses and Working Principle Rectification Definition Chemistry It is used for substances that are required in high purity and/or large quantities, for example to. Rectification is an application of distillation. Rectification denotes a change of concentrations beyond that of simple distillation. The article contains sections titled: Distillation is extensively used in chemical, petrochemical, pharmaceutical. Distillation (or rectification) is the most important technique for separating fluid mixtures into. Rectification Definition Chemistry.
From www.slideshare.net
Physics Rectification Definition Chemistry Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is a very effective separation. Rectification is an application of distillation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. It is used for substances that are required in high purity and/or large quantities, for example to. Distillation (or rectification) is the. Rectification Definition Chemistry.
From en.ppt-online.org
Rectification. Difference between the rectification and distillation Rectification Definition Chemistry The article contains sections titled: Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. It is used for substances that are required in high purity and/or large quantities, for example. Rectification Definition Chemistry.
From www.youtube.com
Fractional distillation Animation striping rectification YouTube Rectification Definition Chemistry Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Rectification is an application of distillation. The article contains sections titled: It is used for substances that are required in high purity and/or large quantities, for example to. When you. Rectification Definition Chemistry.
From www.studypool.com
SOLUTION 10 principles of rectification Studypool Rectification Definition Chemistry The article contains sections titled: When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both ultimately trace back to. Rectification is a very effective separation. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification is an application of distillation. Rectification denotes a change of concentrations beyond that of simple distillation.. Rectification Definition Chemistry.
From www.collegesearch.in
Rectifier Definition, Types, Application, Uses and Working Principle Rectification Definition Chemistry Rectification is an application of distillation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Distillation (or rectification) is the most important technique for separating fluid mixtures into pure substances. It is used for substances that are required in high purity and/or large quantities, for example. Rectification Definition Chemistry.
From studylib.net
DEFINITION rectification is the conversion of alternating current (AC Rectification Definition Chemistry Rectification is a very effective separation. Fractional distillation is often used to separate mixtures of liquids that have similar boiling points. Distillation is extensively used in chemical, petrochemical, pharmaceutical. Rectification denotes a change of concentrations beyond that of simple distillation. When you rectify something, you correct an error or make things right, which is fitting because rectify and correct both. Rectification Definition Chemistry.
From www.chegg.com
Solved 1. Define rectification. 2. What is the main function Rectification Definition Chemistry Distillation is extensively used in chemical, petrochemical, pharmaceutical. The article contains sections titled: Rectification denotes a change of concentrations beyond that of simple distillation. Rectification is a very effective separation. It is used for substances that are required in high purity and/or large quantities, for example to. When you rectify something, you correct an error or make things right, which. Rectification Definition Chemistry.