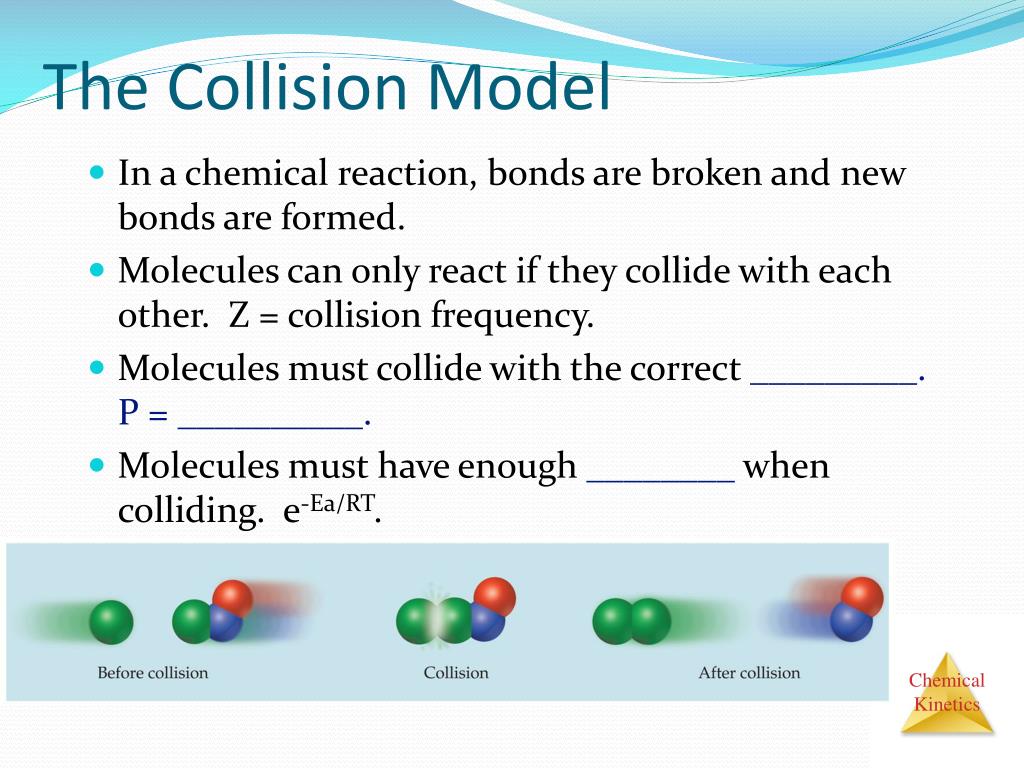
Collision Model Definition . Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. With an increase in the concentration of any reacting. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why most reaction rates increase as concentrations increase.
from www.slideserve.com
Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory states that for a chemical reaction to. With an increase in the concentration of any reacting. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why most reaction rates increase as concentrations increase.
PPT Chapter 13 Chemical PowerPoint Presentation, free
Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any reacting. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory states that for a chemical reaction to. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates.
From www.sciencefacts.net
Elastic Collision Definition, Formula, and Examples Collision Model Definition With an increase in the concentration of any reacting. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory states that for a chemical reaction to. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory, theory used to predict the rates of chemical reactions,. Collision Model Definition.
From studylib.net
Collision Theory and Potential Energy Diagrams Collision Model Definition Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. With an increase in the concentration of any reacting. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many. Collision Model Definition.
From www.slideserve.com
PPT Chapter 14 Chemical PowerPoint Presentation, free Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why most reaction rates increase as concentrations. Collision Model Definition.
From pablogroeaton.blogspot.com
Collision Theory of Reaction Rates PablogroEaton Collision Model Definition With an increase in the concentration of any reacting. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory. Collision Model Definition.
From www.slideserve.com
PPT Chapter 18 PowerPoint Presentation, free download ID6545697 Collision Model Definition Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. With an increase in the concentration of any reacting. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but. Collision Model Definition.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation. Collision Model Definition.
From www.pinterest.com
Collision Theory Easy Science Collision theory, Theories, Theory Collision Model Definition Collision theory explains why most reaction rates increase as concentrations increase. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions,. Collision Model Definition.
From www.slideserve.com
PPT Chapter 13 Chemical PowerPoint Presentation, free Collision Model Definition Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a. Collision Model Definition.
From www.savemyexams.com
Collision Theory Cambridge O Level Chemistry Revision Notes 2023 Collision Model Definition Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any. Collision Model Definition.
From www.slideserve.com
PPT Chapter 18 “Reaction Rates and Equilibrium” PowerPoint Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate. Collision Model Definition.
From www.sciencefacts.net
Inelastic Collision Definition, Formula, and Examples Collision Model Definition Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. With an increase in the concentration of any. Collision Model Definition.
From www.youtube.com
Collision Theory Chemistry Class 12 IIT JEE Main + Advanced Collision Model Definition Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any reacting. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the. Collision Model Definition.
From www.slideshare.net
Collision Theory Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. With an increase in the concentration of any. Collision Model Definition.
From studyfinder.org
Understanding Collision Theory Unraveling Gizmo Answers Collision Model Definition Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for. Collision Model Definition.
From www.youtube.com
6.1 Collision theory (SL) YouTube Collision Model Definition Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters. Collision Model Definition.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory explains why different reactions occur at different rates, and suggests ways. Collision Model Definition.
From chem.libretexts.org
Chapter 14.7 The Collision Model of Chemical Chemistry Collision Model Definition Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a simple but effective explanation. Collision Model Definition.
From byjus.com
Collision Theory Definition, Explanation, Activation energy, Arrhenius Collision Model Definition Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why different reactions occur at different rates, and suggests ways. Collision Model Definition.
From www.geeksforgeeks.org
Elastic Collision Definition, Formula, and Example Collision Model Definition Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any reacting. Collision theory states that. Collision Model Definition.
From www.youtube.com
Collision Theory and How to Ensure Effective Collisions in Chemical Collision Model Definition Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. With an increase in the concentration of any reacting. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. Collision theory provides a. Collision Model Definition.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. With an increase in. Collision Model Definition.
From www.vrogue.co
The Collision Model Of Chemical vrogue.co Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a simple but effective explanation. Collision Model Definition.
From saylordotorg.github.io
The Collision Model of Chemical Collision Model Definition Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any reacting. Collision theory. Collision Model Definition.
From www.sliderbase.com
The Reaction Mechanism Presentation Chemistry Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters. Collision Model Definition.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Model Definition Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why different reactions occur at different rates, and suggests ways to change. Collision Model Definition.
From www.youtube.com
Collision Theory YouTube Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. With an increase in the concentration of any reacting. Collision theory explains why different reactions occur at different rates,. Collision Model Definition.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID3873529 Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why. Collision Model Definition.
From www.slideserve.com
PPT Chemical Chapter 13 PowerPoint Presentation, free Collision Model Definition Collision theory states that for a chemical reaction to. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any reacting. Collision theory provides a. Collision Model Definition.
From lessonmariann.z21.web.core.windows.net
Collision Definition Science Collision Model Definition Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With. Collision Model Definition.
From slidetodoc.com
CHEM 3310 Chemical Collision Theory Transition State Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision. Collision Model Definition.
From wiringfixsprang.z19.web.core.windows.net
Parts Of Collision Theory Collision Model Definition Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation. Collision Model Definition.
From www.stuvia.com
COLLISION THEORY DEFINITION & SIGNIFICANCE WITH VERIFIED SOLUTIONS Collision Model Definition Collision theory states that for a chemical reaction to. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory,. Collision Model Definition.
From www.slideserve.com
PPT Chemistry 232 PowerPoint Presentation, free download ID2214653 Collision Model Definition Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. Collision Model Definition.
From www.slideserve.com
PPT The Collision Model PowerPoint Presentation, free download ID Collision Model Definition With an increase in the concentration of any reacting. Collision theory explains why most reaction rates increase as concentrations increase. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory, theory used to predict the rates of chemical reactions, particularly for gases. Collision theory states that for a chemical reaction. Collision Model Definition.
From www.slideserve.com
PPT Collision Theory and Reaction Rates PowerPoint Presentation, free Collision Model Definition Collision theory states that for a chemical reaction to. Collision theory explains why different reactions occur at different rates, and suggests ways to change the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. With an increase in the concentration of any reacting. Collision theory provides a. Collision Model Definition.