Dilute Hcl Formula . state whether the concentration of a solution is directly or indirectly proportional to its volume. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. you dilute the solution by adding enough water to make the solution volume 500.ml. The new molarity can easily be calculated. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. The solution is diluted by adding enough water to make 500. You can use this calculator to determine how much of it you need if you. write the formula for calculating. suppose there are 100. Ml of a 2.0 m solution of hcl available. imagine you have a concentrated solution of hydrochloric acid.
from www.teachoo.com
The solution is diluted by adding enough water to make 500. you dilute the solution by adding enough water to make the solution volume 500.ml. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. state whether the concentration of a solution is directly or indirectly proportional to its volume. imagine you have a concentrated solution of hydrochloric acid. Ml of a 2.0 m solution of hcl available. write the formula for calculating. The new molarity can easily be calculated. suppose there are 100. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes.
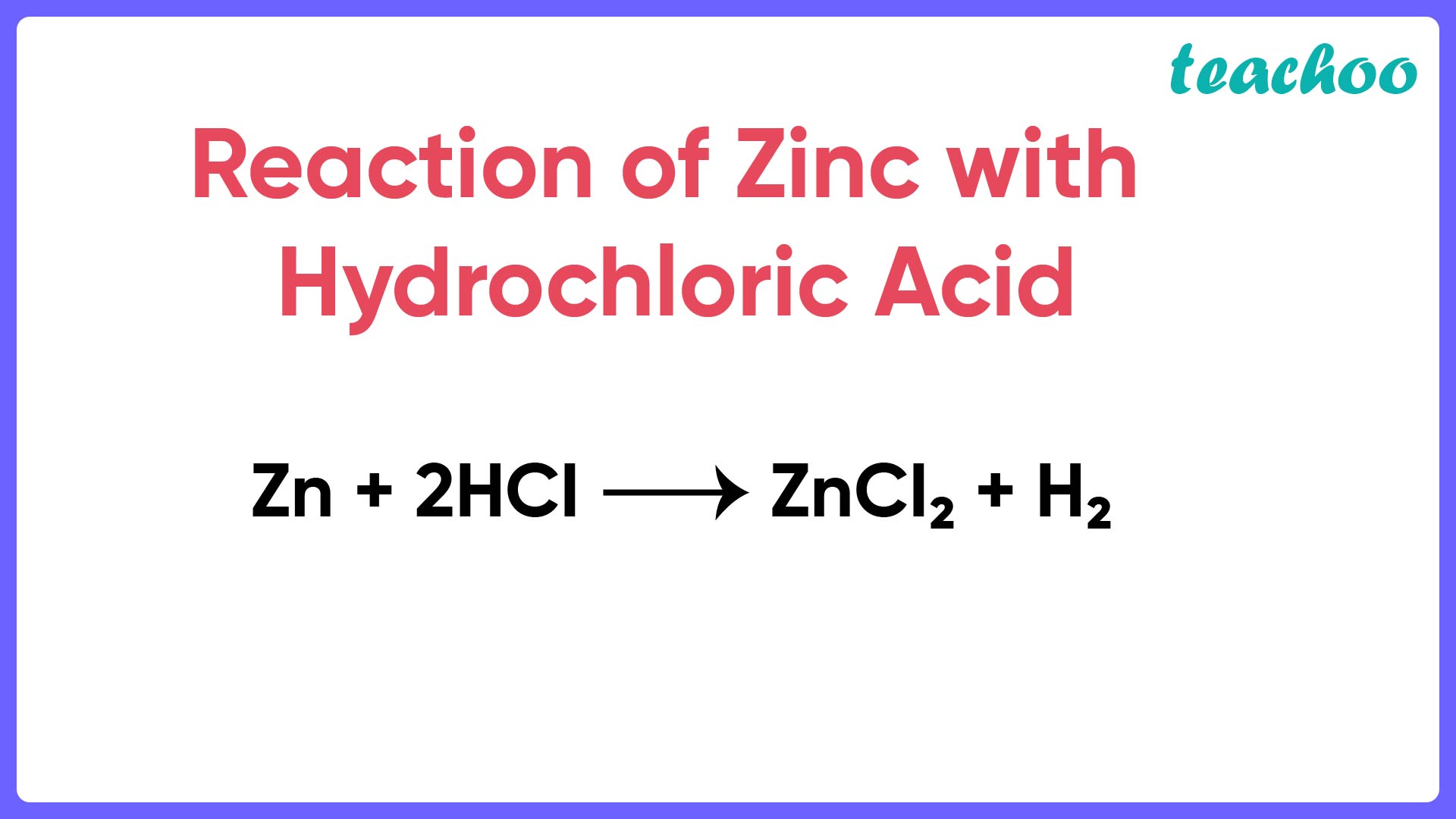
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro
Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. suppose there are 100. You can use this calculator to determine how much of it you need if you. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. imagine you have a concentrated solution of hydrochloric acid. state whether the concentration of a solution is directly or indirectly proportional to its volume. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. The solution is diluted by adding enough water to make 500. Ml of a 2.0 m solution of hcl available. The new molarity can easily be calculated. write the formula for calculating. you dilute the solution by adding enough water to make the solution volume 500.ml.
From guidediagramterrain.z5.web.core.windows.net
Lewis Dot Diagram For Hydrochloric Acid Dilute Hcl Formula The solution is diluted by adding enough water to make 500. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. imagine you have a concentrated solution of hydrochloric acid. suppose there are 100. The new molarity can easily be calculated. Ml of a 2.0 m solution of hcl available. state whether. Dilute Hcl Formula.
From www.toppr.com
Write a balanced chemical equation for the reaction of calcium Dilute Hcl Formula You can use this calculator to determine how much of it you need if you. Ml of a 2.0 m solution of hcl available. write the formula for calculating. imagine you have a concentrated solution of hydrochloric acid. state whether the concentration of a solution is directly or indirectly proportional to its volume. The solution is diluted. Dilute Hcl Formula.
From www.sciencephoto.com
Bottle of dilute hydrochloric acid Stock Image C019/8337 Science Dilute Hcl Formula state whether the concentration of a solution is directly or indirectly proportional to its volume. The new molarity can easily be calculated. write the formula for calculating. you dilute the solution by adding enough water to make the solution volume 500.ml. The solution is diluted by adding enough water to make 500. imagine you have a. Dilute Hcl Formula.
From www.numerade.com
SOLVED write balanced chemical equation for the following reactions a Dilute Hcl Formula imagine you have a concentrated solution of hydrochloric acid. Ml of a 2.0 m solution of hcl available. suppose there are 100. write the formula for calculating. The solution is diluted by adding enough water to make 500. you dilute the solution by adding enough water to make the solution volume 500.ml. state whether the. Dilute Hcl Formula.
From niasrfrank.blogspot.com
Calcium Reaction With Dilute Hydrochloric Acid NiasrFrank Dilute Hcl Formula You can use this calculator to determine how much of it you need if you. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. Ml of a 2.0 m solution of hcl available. imagine you have a concentrated solution of hydrochloric acid. the hcl dilution calculator simplifies this process. Dilute Hcl Formula.
From brainly.in
explain the action of dilute hydrochloric acid on the following with Dilute Hcl Formula imagine you have a concentrated solution of hydrochloric acid. Ml of a 2.0 m solution of hcl available. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. You can use this calculator to determine how much of it you need if you. you dilute the solution by adding enough water to make. Dilute Hcl Formula.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. The new molarity can easily be calculated. suppose there are 100. imagine you have a concentrated solution of hydrochloric acid. Ml of a 2.0 m solution of hcl available. you dilute the solution by adding enough water to make. Dilute Hcl Formula.
From www.reddit.com
Write the net ionic equation in which the slightly soluble salt Dilute Hcl Formula write the formula for calculating. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. suppose there are 100. The solution is diluted by adding enough water to make 500. You can use this calculator to determine how much of it you need if you. Ml of a 2.0 m solution of hcl. Dilute Hcl Formula.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Dilute Hcl Formula imagine you have a concentrated solution of hydrochloric acid. you dilute the solution by adding enough water to make the solution volume 500.ml. suppose there are 100. You can use this calculator to determine how much of it you need if you. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions.. Dilute Hcl Formula.
From www.chegg.com
Solved Dilution Problems For problems 7 11 use the dilution Dilute Hcl Formula Ml of a 2.0 m solution of hcl available. write the formula for calculating. imagine you have a concentrated solution of hydrochloric acid. suppose there are 100. you dilute the solution by adding enough water to make the solution volume 500.ml. state whether the concentration of a solution is directly or indirectly proportional to its. Dilute Hcl Formula.
From www.sarthaks.com
Write balanced equations for the reaction of dilute hydrochloric acid Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. you dilute the solution by adding enough water to make the solution volume 500.ml. The new molarity can easily be calculated. You can use this calculator to determine how much of it you need if you. write the formula for. Dilute Hcl Formula.
From www.sciencephoto.com
Bottle of dilute hydrochloric acid Stock Image C019/8335 Science Dilute Hcl Formula Ml of a 2.0 m solution of hcl available. imagine you have a concentrated solution of hydrochloric acid. state whether the concentration of a solution is directly or indirectly proportional to its volume. The new molarity can easily be calculated. You can use this calculator to determine how much of it you need if you. you dilute. Dilute Hcl Formula.
From www.indiamart.com
Dilute Hydrochloric Acid, For Laboratory, 99 Pure at Rs 300/litre in Dilute Hcl Formula state whether the concentration of a solution is directly or indirectly proportional to its volume. Ml of a 2.0 m solution of hcl available. suppose there are 100. The new molarity can easily be calculated. imagine you have a concentrated solution of hydrochloric acid. write the formula for calculating. the hcl dilution calculator simplifies this. Dilute Hcl Formula.
From www.numerade.com
SOLVED You need to use a dilute hydrochloric acid solution in an Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. you dilute the solution by adding enough water to make the solution volume 500.ml. The solution is diluted by adding enough water to make 500. state whether the concentration of a solution is directly or indirectly proportional to its volume.. Dilute Hcl Formula.
From www.youtube.com
Tin(Sn)+Dilute hydrochloric acid (HCl).The Balanced chemical Equation Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. imagine you have a concentrated solution of hydrochloric acid. suppose there are 100. The new molarity can easily be calculated. write the formula for calculating. The solution is diluted by adding enough water to make 500. you dilute. Dilute Hcl Formula.
From brainly.in
what happen when dilute hydrochloric acid is added to the following Dilute Hcl Formula imagine you have a concentrated solution of hydrochloric acid. write the formula for calculating. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. suppose there are 100. Ml of a 2.0 m solution of hcl available. The solution is diluted by adding enough water to make 500. the formula for. Dilute Hcl Formula.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. The new molarity can easily be calculated. You can use this calculator to determine how much of it you need if you. you dilute the solution by adding enough water to make the solution volume 500.ml. suppose there are 100.. Dilute Hcl Formula.
From byjus.com
which of the following is insoluble in dilute HCl (1)CdCl2 (2)PbCl2 (3 Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. state whether the concentration of a solution is directly or indirectly proportional to its volume. Ml of a 2.0 m solution of hcl available. You can use this calculator to determine how much of it you need if you. the. Dilute Hcl Formula.
From www.gauthmath.com
Solved 12 Magnesium reacts with dilute hydrochloric acid. The equation Dilute Hcl Formula suppose there are 100. The new molarity can easily be calculated. imagine you have a concentrated solution of hydrochloric acid. The solution is diluted by adding enough water to make 500. You can use this calculator to determine how much of it you need if you. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric. Dilute Hcl Formula.
From www.youtube.com
How to prepare dilute solution of hydrochloric acid (HCl) in laboratory Dilute Hcl Formula suppose there are 100. write the formula for calculating. The solution is diluted by adding enough water to make 500. The new molarity can easily be calculated. You can use this calculator to determine how much of it you need if you. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations. Dilute Hcl Formula.
From www.chegg.com
Solved Part 1 Preparation of dilute hydrochloric acid (only Dilute Hcl Formula suppose there are 100. The new molarity can easily be calculated. state whether the concentration of a solution is directly or indirectly proportional to its volume. The solution is diluted by adding enough water to make 500. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. You can use. Dilute Hcl Formula.
From dxoomloun.blob.core.windows.net
Dilute Hcl Preparation at Jesus McLaughlin blog Dilute Hcl Formula imagine you have a concentrated solution of hydrochloric acid. suppose there are 100. You can use this calculator to determine how much of it you need if you. The new molarity can easily be calculated. The solution is diluted by adding enough water to make 500. the formula for diluting hcl solutions involves the relationship between the. Dilute Hcl Formula.
From brainly.in
explain the action of dilute hydrochloric acid on the following with Dilute Hcl Formula the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. write the formula for calculating. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. you dilute the solution by adding enough water to make the solution volume 500.ml. The new molarity can easily be. Dilute Hcl Formula.
From brainly.in
explain the action of dilute hydrochloric acid on the following with Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. write the formula for calculating. Ml of a 2.0 m solution of hcl available. you dilute the solution by adding enough water to make the solution volume 500.ml. The solution is diluted by adding enough water to make 500. . Dilute Hcl Formula.
From www.chegg.com
Solved Part 1 Preparation of dilute hydrochloric acid (only Dilute Hcl Formula The new molarity can easily be calculated. You can use this calculator to determine how much of it you need if you. imagine you have a concentrated solution of hydrochloric acid. write the formula for calculating. Ml of a 2.0 m solution of hcl available. The solution is diluted by adding enough water to make 500. suppose. Dilute Hcl Formula.
From www.youtube.com
How to write the formula for Hydrochloric acid (HCl) YouTube Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. state whether the concentration of a solution is directly or indirectly proportional to its volume. write the formula for calculating. The solution is diluted by adding enough water to make 500. You can use this calculator to determine how much. Dilute Hcl Formula.
From brainly.in
Explain the action of dilute hydrochloric acid on the following with Dilute Hcl Formula imagine you have a concentrated solution of hydrochloric acid. The solution is diluted by adding enough water to make 500. Ml of a 2.0 m solution of hcl available. you dilute the solution by adding enough water to make the solution volume 500.ml. suppose there are 100. the formula for diluting hcl solutions involves the relationship. Dilute Hcl Formula.
From brainly.in
2)What happens when dilute HCl is added to sodium carbonate.? Write a Dilute Hcl Formula write the formula for calculating. imagine you have a concentrated solution of hydrochloric acid. The new molarity can easily be calculated. state whether the concentration of a solution is directly or indirectly proportional to its volume. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. the hcl. Dilute Hcl Formula.
From www.nagwa.com
Question Video Determining Which Anions Can Be Detected Using Dilute Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. Ml of a 2.0 m solution of hcl available. The solution is diluted by adding enough water to make 500. You can use this calculator to determine how much of it you need if you. state whether the concentration of a. Dilute Hcl Formula.
From dxoomloun.blob.core.windows.net
Dilute Hcl Preparation at Jesus McLaughlin blog Dilute Hcl Formula The new molarity can easily be calculated. Ml of a 2.0 m solution of hcl available. imagine you have a concentrated solution of hydrochloric acid. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. The solution is diluted by adding enough water to make 500. You can use this calculator. Dilute Hcl Formula.
From www.numerade.com
SOLVED what happens when dilute HCl is added to iron fillings write Dilute Hcl Formula Ml of a 2.0 m solution of hcl available. write the formula for calculating. you dilute the solution by adding enough water to make the solution volume 500.ml. the hcl dilution calculator simplifies this process specifically for diluting hydrochloric acid (hcl) solutions. The solution is diluted by adding enough water to make 500. imagine you have. Dilute Hcl Formula.
From www.numerade.com
Dilute HCl(a q) and KOH(a q) are mixed in chemically equivalent Dilute Hcl Formula you dilute the solution by adding enough water to make the solution volume 500.ml. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. You can use this calculator to determine how much of it you need if you. Ml of a 2.0 m solution of hcl available. write the. Dilute Hcl Formula.
From www.chegg.com
Part 1 Preparation of dilute hydrochloric acid 1. Dilute Hcl Formula state whether the concentration of a solution is directly or indirectly proportional to its volume. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. The new molarity can easily be calculated. you dilute the solution by adding enough water to make the solution volume 500.ml. imagine you have. Dilute Hcl Formula.
From dxoomloun.blob.core.windows.net
Dilute Hcl Preparation at Jesus McLaughlin blog Dilute Hcl Formula state whether the concentration of a solution is directly or indirectly proportional to its volume. the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. you dilute the solution by adding enough water to make the solution volume 500.ml. suppose there are 100. imagine you have a concentrated. Dilute Hcl Formula.
From exoaldujs.blob.core.windows.net
How To Write Dilutions at Judy Moody blog Dilute Hcl Formula the formula for diluting hcl solutions involves the relationship between the initial and final concentrations and volumes. you dilute the solution by adding enough water to make the solution volume 500.ml. Ml of a 2.0 m solution of hcl available. suppose there are 100. write the formula for calculating. The new molarity can easily be calculated.. Dilute Hcl Formula.