Examples Of Self Indicators In Chemistry . The most common example here is potassium manganate vii. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. The permanganate ion is coloured an intense. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. indicators are substances whose solutions change color due to changes in ph. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and.
from www.slideserve.com
Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The most common example here is potassium manganate vii. The permanganate ion is coloured an intense. indicators are substances whose solutions change color due to changes in ph.
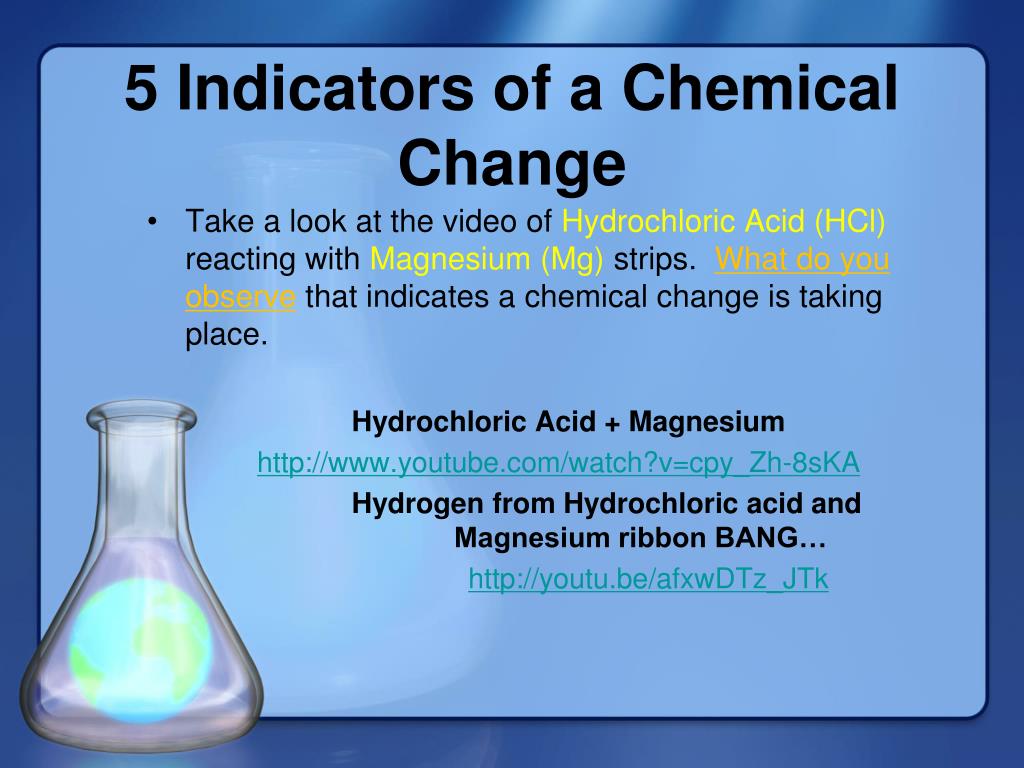
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free
Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The permanganate ion is coloured an intense. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. The most common example here is potassium manganate vii. indicators are substances whose solutions change color due to changes in ph.
From dxofrcamx.blob.core.windows.net
What Is The Meaning Of Indicator In Chemistry at Laura Nelson blog Examples Of Self Indicators In Chemistry Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. indicators are substances whose solutions change color due to changes in ph. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free Examples Of Self Indicators In Chemistry Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. indicators are substances whose solutions change color due to. Examples Of Self Indicators In Chemistry.
From www.storyboardthat.com
Indicators of a Chemical Reaction Storyboard od oliversmith Examples Of Self Indicators In Chemistry the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. The most common example here is potassium manganate vii. The permanganate ion is coloured an intense. indicators are substances whose solutions change color due to changes in ph. chemical indicators create a. Examples Of Self Indicators In Chemistry.
From www.researchgate.net
Percentage Indicators of Chemistry SelfConcept Download Scientific Examples Of Self Indicators In Chemistry indicators are substances whose solutions change color due to changes in ph. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free Examples Of Self Indicators In Chemistry indicators are substances whose solutions change color due to changes in ph. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. The most common example here is potassium manganate vii. the most important class of indicators are substances that do not participate in the. Examples Of Self Indicators In Chemistry.
From www.youtube.com
Self Indicators Redox Titration Analytical Chemistry Internal Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. indicators are substances whose solutions change color due to changes in ph. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic. Examples Of Self Indicators In Chemistry.
From www.chemistry4students.com
Chemistry 4 Students Acid Base indicators Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. indicators are substances whose solutions change color due. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free Examples Of Self Indicators In Chemistry indicators are substances whose solutions change color due to changes in ph. The permanganate ion is coloured an intense. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. the most important class of indicators are substances that do not participate in the redox. Examples Of Self Indicators In Chemistry.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. The most common example here is potassium manganate vii. indicators are substances whose solutions change color due to changes in ph. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a. Examples Of Self Indicators In Chemistry.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. The most common example here is potassium manganate vii. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. the. Examples Of Self Indicators In Chemistry.
From dxokftznc.blob.core.windows.net
Indicators In Chemistry Pdf at Kevin Stovall blog Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. the most important class of indicators are substances that. Examples Of Self Indicators In Chemistry.
From dxojsggnf.blob.core.windows.net
Indicator Chemistry Examples at Gary Stanton blog Examples Of Self Indicators In Chemistry chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. The most common example here is potassium manganate vii. indicators are substances whose solutions change color due to changes in ph. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of. Examples Of Self Indicators In Chemistry.
From www.youtube.com
What are Indicators and how do we use them? The Chemistry Journey Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. indicators are substances whose solutions change color due to changes in ph. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. The most common example here is potassium manganate vii. the most important class of indicators are. Examples Of Self Indicators In Chemistry.
From www.youtube.com
ACID BASE INDICATORS YouTube Examples Of Self Indicators In Chemistry Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. The permanganate ion is coloured an intense. indicators are substances whose solutions change color due to changes in ph. the most important class of indicators are substances that do not participate in the redox. Examples Of Self Indicators In Chemistry.
From exozpofnp.blob.core.windows.net
AcidBase Indicators Definition Chemistry at Tyra Colon blog Examples Of Self Indicators In Chemistry chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The most common example here is potassium manganate vii. The permanganate ion is coloured an intense. indicators are substances whose solutions change color due to changes in ph. Kmno 4 acts as an self indicator during titration between kmno 4 and. Examples Of Self Indicators In Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Examples Of Self Indicators In Chemistry chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The most common example here is potassium manganate vii. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. indicators are substances whose solutions change color due. Examples Of Self Indicators In Chemistry.
From study.com
AcidBase Indicators Uses & Examples Video & Lesson Transcript Examples Of Self Indicators In Chemistry indicators are substances whose solutions change color due to changes in ph. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. The permanganate ion is coloured an intense. The most common example here is potassium manganate vii. the most important class of indicators are. Examples Of Self Indicators In Chemistry.
From www.youtube.com
Trick to Memorize Colours and pH of Indicators Organic Chemistry Examples Of Self Indicators In Chemistry chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. The most common example here is potassium manganate vii. chemical indicators create a visible sign (usually, a. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT Litmus Indicator PowerPoint Presentation, free download ID2790323 Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. indicators are substances whose solutions change. Examples Of Self Indicators In Chemistry.
From vhmsscience8.weebly.com
Chemical & Physical Change Indicators 1 VISTA HEIGHTS 8TH GRADE SCIENCE Examples Of Self Indicators In Chemistry Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. The permanganate ion is coloured an intense. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. indicators are. Examples Of Self Indicators In Chemistry.
From www.youtube.com
Indicators Chemistry 12 Sec 4.17 YouTube Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. The permanganate ion is coloured an intense. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color.. Examples Of Self Indicators In Chemistry.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. The most common example here is potassium manganate vii. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. Kmno 4 acts. Examples Of Self Indicators In Chemistry.
From ccschemistry.blogspot.com
Cowbridge Chemistry Department Indicators Colours and pH ranges Examples Of Self Indicators In Chemistry chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The most common example here is potassium manganate vii. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a visible sign. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free Examples Of Self Indicators In Chemistry the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. The permanganate ion is coloured an intense. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. indicators are substances. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free Examples Of Self Indicators In Chemistry the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. The most common example here is potassium manganate vii. . Examples Of Self Indicators In Chemistry.
From www.savemyexams.com
Indicators Edexcel IGCSE Chemistry Double Science Revision Notes 2019 Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The permanganate ion is coloured an intense. chemical. Examples Of Self Indicators In Chemistry.
From exohsfqml.blob.core.windows.net
Types Of Indicators In Chemistry Class 10 at Deborah Topps blog Examples Of Self Indicators In Chemistry The permanganate ion is coloured an intense. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. indicators are substances whose solutions change color due to changes in ph. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT Development of Chemistry Indicators PowerPoint Presentation, free Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. The permanganate ion is coloured an intense. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. indicators are substances whose solutions change color due to changes in ph. Kmno 4 acts as an. Examples Of Self Indicators In Chemistry.
From www.freepik.com
Premium Vector Chemical reaction indicators. Examples Of Self Indicators In Chemistry chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The permanganate ion is coloured an intense. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a visible sign (usually, a. Examples Of Self Indicators In Chemistry.
From dxojsggnf.blob.core.windows.net
Indicator Chemistry Examples at Gary Stanton blog Examples Of Self Indicators In Chemistry indicators are substances whose solutions change color due to changes in ph. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. The permanganate ion is coloured an intense. The most common example here is potassium manganate vii. chemical indicators play a crucial role in. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT 5 Indicators of a Chemical Change PowerPoint Presentation, free Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The permanganate ion is coloured an intense. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. chemical. Examples Of Self Indicators In Chemistry.
From www.youtube.com
Indicators Definition, Types, Examples and Facts 10th Class Chemistry Examples Of Self Indicators In Chemistry the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. indicators are substances whose solutions change color due to changes in ph. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The most common. Examples Of Self Indicators In Chemistry.
From www.slideserve.com
PPT Chapter 16 AcidBase Equilibria PowerPoint Presentation, free Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. the most important class of indicators are substances that do not participate in the redox titration, but whose oxidized and reduced forms differ in color. chemical indicators create a visible sign (usually, a change in color) during they detect the presence of the threshold concentration of some. chemical. Examples Of Self Indicators In Chemistry.
From www.youtube.com
Chemistry 12 Lesson 22 Indicators YouTube Examples Of Self Indicators In Chemistry The most common example here is potassium manganate vii. indicators are substances whose solutions change color due to changes in ph. Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. the most important class of indicators are substances that do not participate in. Examples Of Self Indicators In Chemistry.
From www.pw.live
Indicators in chemistry Types of Indicators in chemistry Physics Wallah Examples Of Self Indicators In Chemistry Kmno 4 acts as an self indicator during titration between kmno 4 and oxalic acid because it gives pink colour in basic medium and. chemical indicators play a crucial role in chemistry, particularly in understanding the properties of acids and bases. The most common example here is potassium manganate vii. chemical indicators create a visible sign (usually, a. Examples Of Self Indicators In Chemistry.