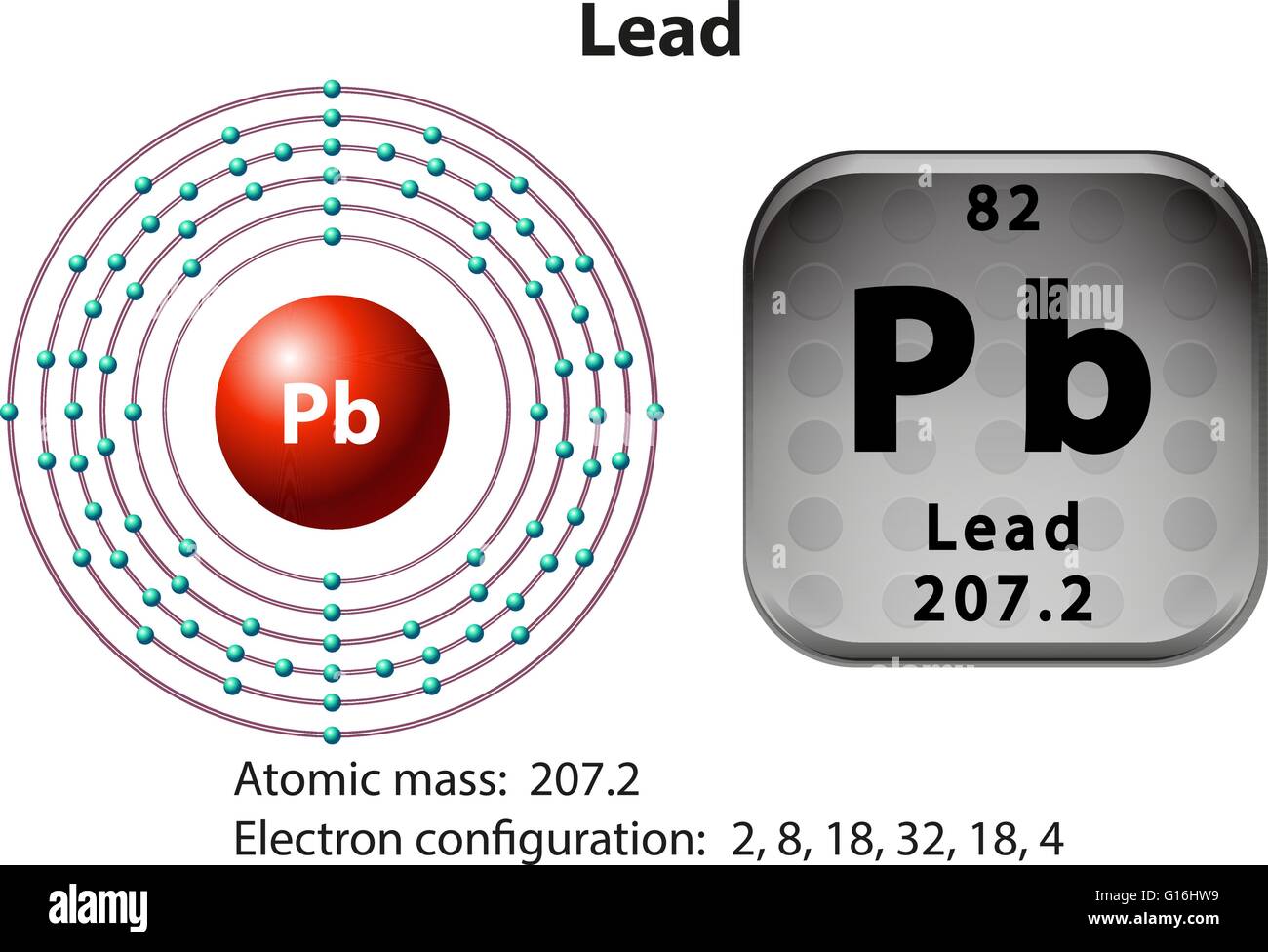
Lead Pb Electrons . 208 pb is the most common isotope, having a natural abundance of approximately 52%. [xe] 6s 2 4f 14 5d 10 6p 2. Relative atomic mass is also known as atomic weight (symbol: Lead electron configuration | image: Lead ion (pb2+, pb4+) electron configuration. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. There are two types of lead ions. Lead occurs in 4 natural isotopes: One is pb 2+ and another is pb 4+. The shorthand electron configuration for lead is: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Lead (pb) has an atomic mass of 82. Find out about its chemical and physical properties, states, energy, electrons,. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the atom.
from www.alamy.com
Relative atomic mass is also known as atomic weight (symbol: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Lead ion (pb2+, pb4+) electron configuration. Lead electron configuration | image: 208 pb is the most common isotope, having a natural abundance of approximately 52%. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. [xe] 6s 2 4f 14 5d 10 6p 2. Lead occurs in 4 natural isotopes: The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the atom. 204 pb, 206 pb, 207 pb and 208 pb.
Atom symbol and electron of lead illustration Stock Vector Image & Art
Lead Pb Electrons Relative atomic mass is also known as atomic weight (symbol: Lead occurs in 4 natural isotopes: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. 204 pb, 206 pb, 207 pb and 208 pb. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the atom. Lead electron configuration | image: The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Lead (pb) has an atomic mass of 82. Lead ion (pb2+, pb4+) electron configuration. There are two types of lead ions. Relative atomic mass is also known as atomic weight (symbol: [xe] 6s 2 4f 14 5d 10 6p 2. 208 pb is the most common isotope, having a natural abundance of approximately 52%. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The shorthand electron configuration for lead is: Find out about its chemical and physical properties, states, energy, electrons,.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead Pb Electrons Lead electron configuration | image: Find out about its chemical and physical properties, states, energy, electrons,. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Lead Pb Electrons.
From material-properties.org
Lead Periodic Table and Atomic Properties Lead Pb Electrons Find out about its chemical and physical properties, states, energy, electrons,. Lead ion (pb2+, pb4+) electron configuration. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. One is pb 2+ and another is pb 4+. Lead electron configuration | image: [xe] 6s 2 4f 14 5d 10 6p 2. Lead occurs in. Lead Pb Electrons.
From www.dreamstime.com
Lead Pb Chemical Element of Periodic Table Isolated on White Background Lead Pb Electrons The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Relative atomic mass is also known as atomic weight (symbol: There are two types of lead ions. Find out about its chemical and physical properties, states, energy, electrons,. Lead electron configuration | image: Lead occurs in 4 natural isotopes: The shorthand. Lead Pb Electrons.
From www.alamy.com
Pb Lead, Periodic Table of the Elements, Shell Structure of Lead Lead Pb Electrons The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the atom. Lead is the 82nd element. Lead Pb Electrons.
From www.istockphoto.com
Pb Lead Element Information Facts Properties Trends Uses And Comparison Lead Pb Electrons Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Relative atomic mass is also known as atomic weight (symbol: 208 pb is the most common isotope, having a natural abundance of approximately 52%. 204 pb, 206 pb, 207 pb and 208 pb. Find out. Lead Pb Electrons.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Pb Electrons Lead occurs in 4 natural isotopes: 208 pb is the most common isotope, having a natural abundance of approximately 52%. [xe] 6s 2 4f 14 5d 10 6p 2. 204 pb, 206 pb, 207 pb and 208 pb. Lead electron configuration | image: The shorthand electron configuration for lead is: There are two types of lead ions. The electron configuration. Lead Pb Electrons.
From periodictableguide.com
Lead (Pb) Periodic Table (Element Information & More) Lead Pb Electrons Lead electron configuration | image: Relative atomic mass is also known as atomic weight (symbol: The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. One is pb 2+ and another is pb 4+. Learn the basics of electron configurations before attempting to write out the configuration for any specific element.. Lead Pb Electrons.
From www.shutterstock.com
Lead Pb Number 82 Element Periodic Stock Vector (Royalty Free Lead Pb Electrons 204 pb, 206 pb, 207 pb and 208 pb. Lead electron configuration | image: [xe] 6s 2 4f 14 5d 10 6p 2. Find out about its chemical and physical properties, states, energy, electrons,. Lead occurs in 4 natural isotopes: Lead (pb) has an atomic mass of 82. There are two types of lead ions. One is pb 2+ and. Lead Pb Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Pb Electrons Relative atomic mass is also known as atomic weight (symbol: The shorthand electron configuration for lead is: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Find out about its chemical and physical properties, states, energy, electrons,. 204 pb, 206 pb, 207 pb and. Lead Pb Electrons.
From www.istockphoto.com
Pb Lead Element Information Facts Properties Trends Uses And Comparison Lead Pb Electrons [xe] 6s 2 4f 14 5d 10 6p 2. Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Lead (pb) has an atomic mass of 82. 204 pb, 206 pb, 207 pb and 208 pb. Lead electron configuration | image: The shorthand electron configuration. Lead Pb Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Pb Electrons There are two types of lead ions. Lead electron configuration | image: Lead occurs in 4 natural isotopes: One is pb 2+ and another is pb 4+. Lead (pb) has an atomic mass of 82. 208 pb is the most common isotope, having a natural abundance of approximately 52%. The shorthand electron configuration for lead is: [xe] 6s 2 4f. Lead Pb Electrons.
From www.alamy.com
Lead (Pb) symbol chemical element of the periodic table, 3D animation Lead Pb Electrons The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. [xe] 6s 2 4f 14 5d 10 6p 2. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Lead is the 82nd element of the periodic table and its atomic number is 82,. Lead Pb Electrons.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead Pb Electrons There are two types of lead ions. Relative atomic mass is also known as atomic weight (symbol: The shorthand electron configuration for lead is: 208 pb is the most common isotope, having a natural abundance of approximately 52%. One is pb 2+ and another is pb 4+. [xe] 6s 2 4f 14 5d 10 6p 2. Lead ion (pb2+, pb4+). Lead Pb Electrons.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Image & Art Lead Pb Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. One is pb 2+ and another is pb 4+. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Lead occurs in 4 natural isotopes: [xe] 6s 2 4f 14 5d 10 6p 2.. Lead Pb Electrons.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Pb Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. Relative atomic mass is also known as atomic weight (symbol: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. One is pb 2+ and another is pb 4+. [xe] 6s. Lead Pb Electrons.
From sciencenotes.org
Lead Facts Pb or Element Number 82 Lead Pb Electrons Lead electron configuration | image: [xe] 6s 2 4f 14 5d 10 6p 2. Relative atomic mass is also known as atomic weight (symbol: Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has. Lead Pb Electrons.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Pb Electrons There are two types of lead ions. 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. [xe] 6s 2 4f 14. Lead Pb Electrons.
From www.alamy.com
Lead (Pb). Diagram of the nuclear composition, electron configuration Lead Pb Electrons Learn the basics of electron configurations before attempting to write out the configuration for any specific element. The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d. Lead Pb Electrons.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead Pb Electrons The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the atom. Lead ion (pb2+, pb4+) electron. Lead Pb Electrons.
From www.alamy.com
Pb Lead Chemical Element Periodic Table. Single vector illustration Lead Pb Electrons Relative atomic mass is also known as atomic weight (symbol: The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Find out about its chemical and physical properties, states, energy, electrons,. 204 pb, 206 pb, 207 pb and 208 pb. There are two types of lead ions. One is pb 2+. Lead Pb Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead Pb Electrons Lead occurs in 4 natural isotopes: The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the. Lead Pb Electrons.
From www.shutterstock.com
Lead Pb Chemical Element Vector Illustration Stock Vector (Royalty Free Lead Pb Electrons There are two types of lead ions. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Find out about its chemical and physical properties, states, energy, electrons,. One is pb 2+ and another is pb 4+. Lead electron configuration | image: Learn the basics of electron configurations before attempting to. Lead Pb Electrons.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Pb Electrons [xe] 6s 2 4f 14 5d 10 6p 2. Lead electron configuration | image: Find out about its chemical and physical properties, states, energy, electrons,. One is pb 2+ and another is pb 4+. Learn the basics of electron configurations before attempting to write out the configuration for any specific element. There are two types of lead ions. 204 pb,. Lead Pb Electrons.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Pb Electrons One is pb 2+ and another is pb 4+. 208 pb is the most common isotope, having a natural abundance of approximately 52%. There are two types of lead ions. Lead occurs in 4 natural isotopes: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground. Lead Pb Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead Pb Electrons The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. There are two types of lead ions. Lead occurs in 4 natural isotopes: One is pb 2+ and another is pb 4+. Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has. Lead Pb Electrons.
From periodictable.me
Lead Valence Electrons Lead Valency (Pb) with Dot Diagram Lead Pb Electrons The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise arrangement of electrons within the atom. Find out about its chemical. Lead Pb Electrons.
From www.americanelements.com
Lead (Pb) AMERICAN ELEMENTS Lead Pb Electrons There are two types of lead ions. Lead electron configuration | image: Learn the basics of electron configurations before attempting to write out the configuration for any specific element. Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. One is pb 2+ and another. Lead Pb Electrons.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead Pb Electrons Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Lead (pb) has an atomic mass of 82. There are two types of lead ions. [xe] 6s 2 4f 14 5d 10 6p 2. Lead ion (pb2+, pb4+) electron configuration. Lead occurs in 4 natural. Lead Pb Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead Pb Electrons [xe] 6s 2 4f 14 5d 10 6p 2. Lead (pb) has an atomic mass of 82. 204 pb, 206 pb, 207 pb and 208 pb. 208 pb is the most common isotope, having a natural abundance of approximately 52%. Lead occurs in 4 natural isotopes: Find out about its chemical and physical properties, states, energy, electrons,. Lead electron configuration. Lead Pb Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead Pb Electrons 204 pb, 206 pb, 207 pb and 208 pb. Lead electron configuration | image: Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. The shorthand electron configuration for lead is: The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p. Lead Pb Electrons.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Pb Electrons [xe] 6s 2 4f 14 5d 10 6p 2. Lead electron configuration | image: 208 pb is the most common isotope, having a natural abundance of approximately 52%. Lead (pb) has an atomic mass of 82. Relative atomic mass is also known as atomic weight (symbol: Lead is the 82nd element of the periodic table and its atomic number is. Lead Pb Electrons.
From www.dreamstime.com
Lead, Pb, Periodic Table Element Stock Illustration Illustration of Lead Pb Electrons Lead is the 82nd element of the periodic table and its atomic number is 82, which means it has 82 electrons in its ground state. Lead (pb) has an atomic mass of 82. Lead ion (pb2+, pb4+) electron configuration. Lead electron configuration | image: The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or. Lead Pb Electrons.
From www.shutterstock.com
Lead Pb Element Periodic Table Flat vector de stock (libre de regalías Lead Pb Electrons Find out about its chemical and physical properties, states, energy, electrons,. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The shorthand electron configuration for lead is: The lead electron configuration, represented as [xe] 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s. Lead Pb Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead Pb Electrons 208 pb is the most common isotope, having a natural abundance of approximately 52%. There are two types of lead ions. Lead occurs in 4 natural isotopes: The shorthand electron configuration for lead is: 204 pb, 206 pb, 207 pb and 208 pb. Lead electron configuration | image: Lead (pb) has an atomic mass of 82. Find out about its. Lead Pb Electrons.
From scienceinfo.com
Lead (Pb) Element Properties, Reactions, Uses Lead Pb Electrons One is pb 2+ and another is pb 4+. Lead electron configuration | image: 208 pb is the most common isotope, having a natural abundance of approximately 52%. Lead (pb) has an atomic mass of 82. There are two types of lead ions. Find out about its chemical and physical properties, states, energy, electrons,. Lead is the 82nd element of. Lead Pb Electrons.