Phenolphthalein Is Acidic Or Basic Indicator . Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator of a solution’s ph,. When the number of moles of added base is equal to the number of moles of added. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. Is phenolphthalein acidic or basic? In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can be used to determine the ph of a.
from www.numerade.com
Substances such as phenolphthalein, which can be used to determine the ph of a. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. When the number of moles of added base is equal to the number of moles of added. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. Is phenolphthalein acidic or basic? As an indicator of a solution’s ph,. In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink.
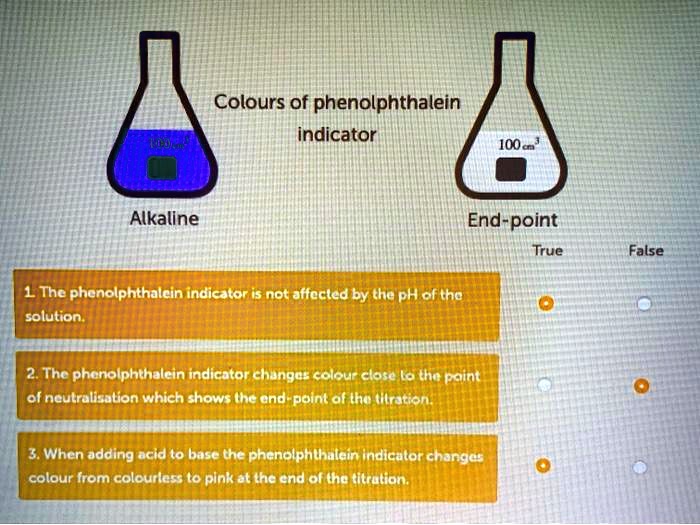
SOLVED Colours of phenolphthalein indicator Alkaline Endpoint True
Phenolphthalein Is Acidic Or Basic Indicator As an indicator of a solution’s ph,. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. When the number of moles of added base is equal to the number of moles of added. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Is phenolphthalein acidic or basic? As an indicator of a solution’s ph,. In this case, the weak acid. In this case, the weak acid is colourless and its ion is bright pink.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Phenolphthalein Is Acidic Or Basic Indicator Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. As an indicator of a solution’s ph,. When the number. Phenolphthalein Is Acidic Or Basic Indicator.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 Phenolphthalein Is Acidic Or Basic Indicator Substances such as phenolphthalein, which can be used to determine the ph of a. In this case, the weak acid. Is phenolphthalein acidic or basic? In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is another commonly used indicator for titrations, and is. Phenolphthalein Is Acidic Or Basic Indicator.
From slideplayer.com
Two Types Of Chemical Reactions ppt download Phenolphthalein Is Acidic Or Basic Indicator Is phenolphthalein acidic or basic? Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. In this case, the weak acid. As an indicator of a solution’s ph,. When the number of moles of added base is equal to the number. Phenolphthalein Is Acidic Or Basic Indicator.
From www.nagwa.com
Question Video Determining the Color of the Indicator Phenolphthalein Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. As an indicator of a solution’s ph,. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is fuchsia in ph's roughly between 8.2. Phenolphthalein Is Acidic Or Basic Indicator.
From www.youtube.com
phenolphthalein turns pink in basic medium and colourless in acidic Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid. In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph. Phenolphthalein Is Acidic Or Basic Indicator.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C030/7311 Science Photo Library Phenolphthalein Is Acidic Or Basic Indicator Substances such as phenolphthalein, which can be used to determine the ph of a. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. As an indicator of a solution’s ph,. In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations,. Phenolphthalein Is Acidic Or Basic Indicator.
From www.slideserve.com
PPT pH—Scale to measure how acidic or basic a solution is 014 Phenolphthalein Is Acidic Or Basic Indicator As an indicator of a solution’s ph,. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Substances such as phenolphthalein, which can be used to determine the ph of a. Is phenolphthalein acidic or basic? In more basic solutions where the hydronium. Phenolphthalein Is Acidic Or Basic Indicator.
From sciencenotes.org
Phenolphthalein Indicator Phenolphthalein Is Acidic Or Basic Indicator Substances such as phenolphthalein, which can be used to determine the ph of a. In this case, the weak acid is colourless and its ion is bright pink. Is phenolphthalein acidic or basic? In this case, the weak acid. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations,. Phenolphthalein Is Acidic Or Basic Indicator.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Phenolphthalein Is Acidic Or Basic Indicator Is phenolphthalein acidic or basic? Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a. In. Phenolphthalein Is Acidic Or Basic Indicator.
From www.numerade.com
SOLVED Colours of phenolphthalein indicator Alkaline Endpoint True Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. When the number of moles of added base is equal to the number of moles of added. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is. Phenolphthalein Is Acidic Or Basic Indicator.
From www.slideserve.com
PPT Chapter 13 Applications of Aqueous Equilibria PowerPoint Phenolphthalein Is Acidic Or Basic Indicator As an indicator of a solution’s ph,. Is phenolphthalein acidic or basic? Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12,. Phenolphthalein Is Acidic Or Basic Indicator.
From mrehingerschemistry.ehinger.nu
6.1. Acids Mr. Ehinger's Chemistry Phenolphthalein Is Acidic Or Basic Indicator When the number of moles of added base is equal to the number of moles of added. In this case, the weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. In this case, the weak acid is. Phenolphthalein Is Acidic Or Basic Indicator.
From slideplayer.com
Chemical/Physical Properties & pH ppt download Phenolphthalein Is Acidic Or Basic Indicator When the number of moles of added base is equal to the number of moles of added. In this case, the weak acid. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. As an indicator of a solution’s ph,. In this case, the weak. Phenolphthalein Is Acidic Or Basic Indicator.
From www.nagwa.com
Question Video Identifying the Color of a Solution Containing the Acid Phenolphthalein Is Acidic Or Basic Indicator In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. As an indicator of a solution’s ph,. Phenolphthalein is another commonly used indicator for titrations,. Phenolphthalein Is Acidic Or Basic Indicator.
From www.compoundchem.com
Compound Interest The Colours & Chemistry of pH Indicators Phenolphthalein Is Acidic Or Basic Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. When the number of moles of added base is equal to the number of moles of added. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. As an indicator of a solution’s ph,. In this case, the weak acid. In. Phenolphthalein Is Acidic Or Basic Indicator.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Phenolphthalein Is Acidic Or Basic Indicator In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. When the number of moles of added base is equal to the number of moles of added. Is phenolphthalein. Phenolphthalein Is Acidic Or Basic Indicator.
From www.vectorstock.com
Phenolphthalein indicator in acidbase titration Vector Image Phenolphthalein Is Acidic Or Basic Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. Is phenolphthalein acidic or basic? Substances such as phenolphthalein, which can be used to determine the ph of a. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is. Phenolphthalein Is Acidic Or Basic Indicator.
From www.youtube.com
Phenolphthalein Indicator Viva Questions pH range and Chemical Phenolphthalein Is Acidic Or Basic Indicator As an indicator of a solution’s ph,. In this case, the weak acid. Is phenolphthalein acidic or basic? Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is fuchsia in. Phenolphthalein Is Acidic Or Basic Indicator.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C039/1218 Science Photo Library Phenolphthalein Is Acidic Or Basic Indicator When the number of moles of added base is equal to the number of moles of added. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used. Phenolphthalein Is Acidic Or Basic Indicator.
From www.sciencephoto.com
Phenolphthalein Indicator Stock Image C039/1215 Science Photo Library Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid. As an indicator of a solution’s ph,. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. When the number of moles of added base is equal to the number of moles of added. In more basic solutions where the hydronium ion concentration is less than 5.0 ×. Phenolphthalein Is Acidic Or Basic Indicator.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Phenolphthalein Is Acidic Or Basic Indicator When the number of moles of added base is equal to the number of moles of added. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. Is phenolphthalein acidic or basic? In more basic solutions where the hydronium ion. Phenolphthalein Is Acidic Or Basic Indicator.
From slideplayer.com
Acidic, Basic, or Neutral Solution ppt download Phenolphthalein Is Acidic Or Basic Indicator Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. As an indicator of a solution’s ph,. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Is phenolphthalein acidic or basic? When the number of moles of added. Phenolphthalein Is Acidic Or Basic Indicator.
From www.shalom-education.com
Indicators Edexcel GCSE Chemistry Revision Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is another commonly used indicator for. Phenolphthalein Is Acidic Or Basic Indicator.
From www.youtube.com
How to make a Phenolphthalein Indicator Solution (0.05wt) YouTube Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid is colourless and its ion is bright pink. In this case, the weak acid. Is phenolphthalein acidic or basic? Substances such as phenolphthalein, which can be used to determine the ph of a. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it. Phenolphthalein Is Acidic Or Basic Indicator.
From stock.adobe.com
Acidbase titration and phenolphthalein indicator Stock Vector Adobe Phenolphthalein Is Acidic Or Basic Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. As an indicator of a solution’s ph,. Is phenolphthalein acidic or basic? In more basic solutions where the. Phenolphthalein Is Acidic Or Basic Indicator.
From avopix.com
Phenolphthalein indicator in acidbase titration Royalty Free Stock Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid. In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Is phenolphthalein acidic or basic? Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12,. Phenolphthalein Is Acidic Or Basic Indicator.
From www.alamy.com
Phenolphthalein acidbase indicator. Phenolphthalein indicator changes Phenolphthalein Is Acidic Or Basic Indicator Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid is colourless and its ion is bright pink. As an indicator of a solution’s ph,. Substances such as phenolphthalein, which can be used to determine the ph of a. In more basic solutions where the hydronium ion concentration is less than. Phenolphthalein Is Acidic Or Basic Indicator.
From mavink.com
Phenolphthalein Ph Range Phenolphthalein Is Acidic Or Basic Indicator As an indicator of a solution’s ph,. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. In this case, the weak acid. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is another commonly used indicator. Phenolphthalein Is Acidic Or Basic Indicator.
From poe.com
What are the colors of Phenolphthalein in acidic and basic solutions? Poe Phenolphthalein Is Acidic Or Basic Indicator As an indicator of a solution’s ph,. In this case, the weak acid. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. When the number of moles of added base is equal to the number of moles of added.. Phenolphthalein Is Acidic Or Basic Indicator.
From www.slideserve.com
PPT Unit 6 Chpt 15 Acid/Base Equilibria PowerPoint Presentation Phenolphthalein Is Acidic Or Basic Indicator In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this case, the weak acid is colourless and its ion is bright pink. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. Phenolphthalein is another commonly used indicator for titrations,. Phenolphthalein Is Acidic Or Basic Indicator.
From www.slideshare.net
P h indicators Phenolphthalein Is Acidic Or Basic Indicator In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. Phenolphthalein is another commonly used indicator for titrations, and is another weak acid. When the number of moles of. Phenolphthalein Is Acidic Or Basic Indicator.
From www.researchgate.net
2 Phenolphthalein Mechanism at Different pH Download Scientific Diagram Phenolphthalein Is Acidic Or Basic Indicator Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. Substances such as phenolphthalein, which can be used to determine the ph of a. Is phenolphthalein acidic or basic?. Phenolphthalein Is Acidic Or Basic Indicator.
From www.vectorstock.com
Phenolphthalein in solutions with different ph Vector Image Phenolphthalein Is Acidic Or Basic Indicator In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this case, the weak acid is colourless and its ion is bright pink. Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is another commonly used indicator for. Phenolphthalein Is Acidic Or Basic Indicator.
From slideplayer.com
Unit Twelve Acids and Bases ppt download Phenolphthalein Is Acidic Or Basic Indicator Substances such as phenolphthalein, which can be used to determine the ph of a. Phenolphthalein is fuchsia in ph's roughly between 8.2 and 12, and is colorless below ph 8.2. In this case, the weak acid. As an indicator of a solution’s ph,. In this case, the weak acid is colourless and its ion is bright pink. In more basic. Phenolphthalein Is Acidic Or Basic Indicator.
From www.youtube.com
Acid Base Indicator Phenolphthalein explained with experiment Phenolphthalein Is Acidic Or Basic Indicator In this case, the weak acid is colourless and its ion is bright pink. In more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or pink. In this case, the weak acid. Is phenolphthalein acidic or basic? Substances such as phenolphthalein, which can be used to determine. Phenolphthalein Is Acidic Or Basic Indicator.