Calorimetry Meaning . calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimeters have been designed in great variety. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Learn how calorimeters work and how to calculate heat transfer using calorimetry. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. what is calorimetry?
from www.indirectcalorimetry.net
Calorimeters have been designed in great variety. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Learn about different types of. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Learn how calorimeters work and how to calculate heat transfer using calorimetry.
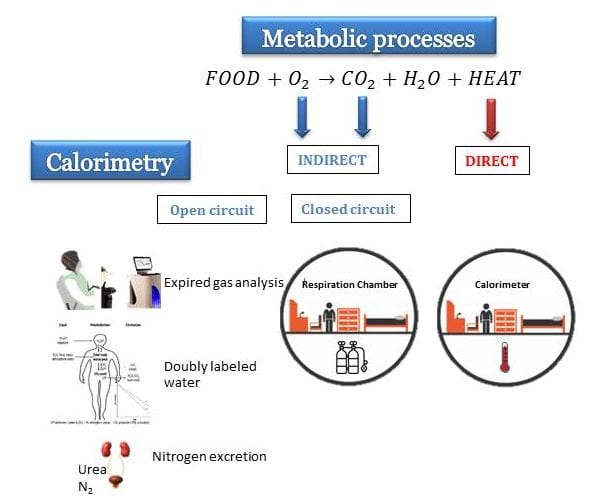
Understanding Indirect Calorimetry Indirect Calorimetry
Calorimetry Meaning Calorimeters have been designed in great variety. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimeters have been designed in great variety. what is calorimetry? calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how calorimeters work and how to calculate heat transfer using calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Learn about different types of. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.
From studylib.net
Calorimetry Calorimetry Meaning calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn how calorimeters work and how to calculate heat transfer using calorimetry. calorimetry is the process of measuring the amount of. Calorimetry Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Meaning calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and. Calorimetry Meaning.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimetry Meaning Learn how calorimeters work and how to calculate heat transfer using calorimetry. Learn about different types of. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters have been designed in great variety. To determine the. Calorimetry Meaning.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Meaning Learn how calorimeters work and how to calculate heat transfer using calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. calorimetry is the set of techniques used. Calorimetry Meaning.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Meaning Calorimeters have been designed in great variety. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Learn how calorimeters work and how to calculate heat transfer using calorimetry. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of. . Calorimetry Meaning.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry Meaning calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. what is calorimetry? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimeters have been designed in great variety. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Learn. Calorimetry Meaning.
From www.slideshare.net
Lesson Enthalpy and Calorimetry Calorimetry Meaning calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimetry is a field of. Calorimetry Meaning.
From www.science-revision.co.uk
Calorimetry Calorimetry Meaning what is calorimetry? calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed in great variety. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry. Calorimetry Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Meaning Learn about different types of. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is a field of thermochemistry that measures the amount of. Calorimetry Meaning.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Meaning Learn how calorimeters work and how to calculate heat transfer using calorimetry. what is calorimetry? calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimetry is the experimental science of measuring. Calorimetry Meaning.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Meaning calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Learn about different types of. calorimetry. Calorimetry Meaning.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Meaning Calorimeters have been designed in great variety. calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimetry Meaning.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimetry Meaning Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the set of techniques used to measure enthalpy changes during chemical. Calorimetry Meaning.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube Calorimetry Meaning Calorimeters have been designed in great variety. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical. Calorimetry Meaning.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Meaning calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Calorimeters have been designed in great variety. what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is a method of measuring the. Calorimetry Meaning.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Calorimetry Meaning To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the set of techniques. Calorimetry Meaning.
From www.youtube.com
Calorimetry Meaning with PronunciationGoogul Dictionary YouTube Calorimetry Meaning Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as. Calorimetry Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Meaning calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. what is calorimetry? calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. Calorimetry Meaning.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Meaning calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Learn how calorimeters work and how to calculate heat transfer using calorimetry. calorimetry is the experimental science of measuring the heat changes that accompany chemical or. Calorimetry Meaning.
From askfilo.com
Calorimetry Meaning, Specific heat capacities, Principle of heat capaci.. Calorimetry Meaning Calorimeters have been designed in great variety. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is a field of thermochemistry that measures the amount of. Calorimetry Meaning.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Calorimetry Meaning calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Learn about different types of. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Calorimetry Meaning.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Meaning Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such. Calorimetry Meaning.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Meaning Calorimeters have been designed in great variety. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Learn about different types of. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Learn how calorimeters work and how to calculate heat transfer using calorimetry. what is calorimetry?. Calorimetry Meaning.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Meaning Learn about different types of. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry. Calorimetry Meaning.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Meaning calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Learn how calorimeters work and how to calculate heat transfer using calorimetry. Calorimeters have been designed in great variety. calorimeter,. Calorimetry Meaning.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimetry Meaning calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of. what is calorimetry? Calorimeters have been designed in great variety. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Learn. Calorimetry Meaning.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Calorimetry Meaning calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such as phase changes. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. Learn about. Calorimetry Meaning.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Meaning To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. what is calorimetry? Learn about different types of. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is a. Calorimetry Meaning.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Meaning Learn how calorimeters work and how to calculate heat transfer using calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter,. Calorimetry Meaning.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetry Meaning Learn about different types of. Learn how calorimeters work and how to calculate heat transfer using calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. Calorimetry Meaning.
From gamesmartz.com
Calorimetry Definition & Image GameSmartz Calorimetry Meaning calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its. Calorimetry Meaning.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Meaning Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. calorimeter, device for. Calorimetry Meaning.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Meaning Learn about different types of. calorimetry is the experimental science of measuring the heat changes that accompany chemical or physical changes. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. Calorimetry Meaning.
From www.indirectcalorimetry.net
Understanding Indirect Calorimetry Indirect Calorimetry Calorimetry Meaning calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a method of measuring the heat energy involved in chemical reactions or physical processes, such. Calorimetry Meaning.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Meaning Learn how calorimeters work and how to calculate heat transfer using calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimeters have been designed in great variety. Learn about different types of. calorimetry is the process of measuring the amount of heat released. Calorimetry Meaning.