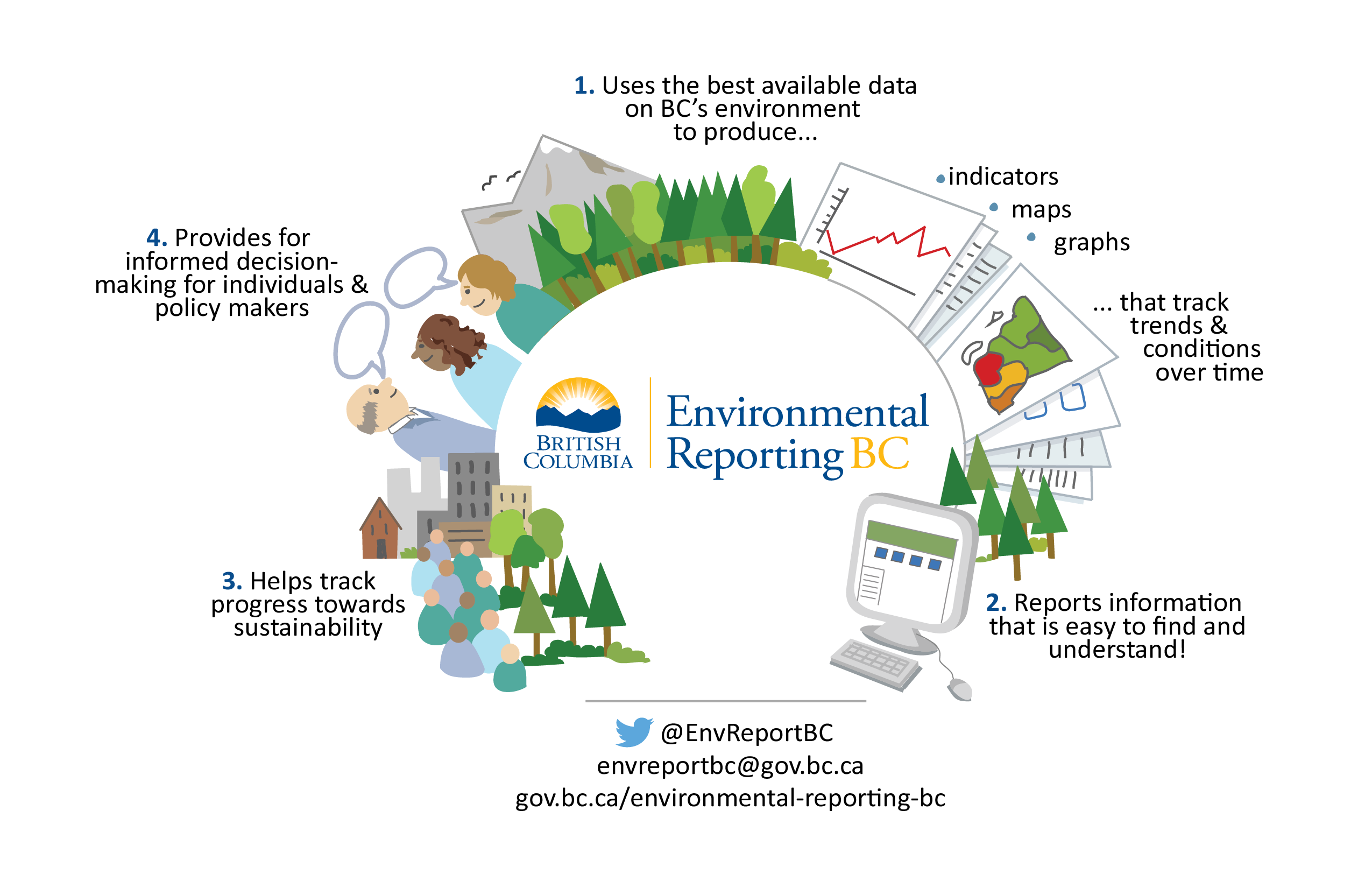
Environmental Monitoring Pics Guidelines . Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. In those cases where parametric release has been.
from www2.gov.bc.ca
This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. In those cases where parametric release has been.
Reporting Province of British Columbia
Environmental Monitoring Pics Guidelines This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. In those cases where parametric release has been. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test.
From studylib.net
environmental management and monitoring plan framework Environmental Monitoring Pics Guidelines This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric. Environmental Monitoring Pics Guidelines.
From sitemate.com
Environmental management plan examples Here's what yours needs Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the. Environmental Monitoring Pics Guidelines.
From www.ifsqn.com
Effective Environmental Monitoring BRC IFSQN Environmental Monitoring Pics Guidelines Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric release has been. This guideline provides general guidance that should be used in the design and. Environmental Monitoring Pics Guidelines.
From www.researchgate.net
(PDF) Environmental monitoring risk assessment Environmental Monitoring Pics Guidelines Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric release has. Environmental Monitoring Pics Guidelines.
From www.researchgate.net
(PDF) Environmental Monitoring and Risk Assessment of Cleanrooms within Environmental Monitoring Pics Guidelines Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here.. Environmental Monitoring Pics Guidelines.
From www.researchgate.net
(PDF) Environmental monitoring a practical approach Environmental Monitoring Pics Guidelines Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. This guideline provides general guidance that should be used in. Environmental Monitoring Pics Guidelines.
From www.researchgate.net
General architecture of an environmental monitoring system based on Environmental Monitoring Pics Guidelines Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. This guideline provides general guidance that should. Environmental Monitoring Pics Guidelines.
From www.pharmaguideline.co.uk
Environmental Monitoring Procedure and its Sop Environmental Monitoring Pics Guidelines This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in. Environmental Monitoring Pics Guidelines.
From nodeviation.com
Environmental Monitoring in Pharma Manufacturing with Mirrhia No Environmental Monitoring Pics Guidelines Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. In those cases where parametric release has. Environmental Monitoring Pics Guidelines.
From studylib.net
Template EP06 Environmental Monitoring and Evaluation of Environmental Monitoring Pics Guidelines Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric release has. Environmental Monitoring Pics Guidelines.
From sitemate.com
Environmental management plan examples Here's what yours needs Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Environmental monitoring, or negative product controls. Environmental Monitoring Pics Guidelines.
From www2.gov.bc.ca
Reporting Province of British Columbia Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental and process monitoring this section differs from guidance given in section. Environmental Monitoring Pics Guidelines.
From www.ppsthane.com
Environmental Monitoring Methods Perfect Pollucon Services Environmental Monitoring Pics Guidelines This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. In those cases where parametric release has been. Pharmacopoeial methods should be used for the validation and performance of the sterility test.. Environmental Monitoring Pics Guidelines.
From www.omnisci.com
What is Environmental Monitoring? Definition and FAQs OmniSci Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. This guideline provides general guidance that should be used in the design and control. Environmental Monitoring Pics Guidelines.
From encyclopedia.pub
Environmental Monitoring Applications Encyclopedia MDPI Environmental Monitoring Pics Guidelines In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Pharmacopoeial methods should be used for the validation and performance of the sterility. Environmental Monitoring Pics Guidelines.
From atlas-scientific.com
5 Types Of Environmental Monitoring Atlas Scientific Environmental Monitoring Pics Guidelines Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. In those cases where parametric release has been. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. This guideline provides general guidance that should be used in the design and. Environmental Monitoring Pics Guidelines.
From www.outsourcedpharma.com
Best Practices In Environmental Monitoring Sampling — Transportation Environmental Monitoring Pics Guidelines In those cases where parametric release has been. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production. Environmental Monitoring Pics Guidelines.
From www.researchgate.net
4 The steps required in a typical environmental impact assessment Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. This guideline provides general guidance that should be used in the design and control. Environmental Monitoring Pics Guidelines.
From www.youtube.com
How to Use the Environmental Monitoring Tool to Manage Environmental Environmental Monitoring Pics Guidelines Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Qualification, cleanroom design, process design, quality control,. Environmental Monitoring Pics Guidelines.
From studylib.net
GUIDELINES ON TEST METHODS FOR ENVIRONMENTAL MONITORING FOR ASEPTIC Environmental Monitoring Pics Guidelines In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental monitoring,. Environmental Monitoring Pics Guidelines.
From digital.library.unt.edu
Environmental Monitoring Plan, Revision 6 UNT Digital Library Environmental Monitoring Pics Guidelines Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental and process monitoring this section differs from. Environmental Monitoring Pics Guidelines.
From www.datacenter-serverroom.com
Data Center & Server Room Environmental Monitoring Environmental Monitoring Pics Guidelines In those cases where parametric release has been. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Pharmacopoeial methods should be used for the validation and performance of the sterility test. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental monitoring, or negative. Environmental Monitoring Pics Guidelines.
From www.sketchbubble.com
Environmental Monitoring PowerPoint Template PPT Slides Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental monitoring, or negative product controls may contribute to the verification of. Environmental Monitoring Pics Guidelines.
From www.youtube.com
ESC Monitoring Equipment Environmental Monitoring Requirements Series Environmental Monitoring Pics Guidelines Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Pharmacopoeial methods should be. Environmental Monitoring Pics Guidelines.
From theintelligentminer.com
Measuring & monitoring mine environmental performance The Intelligent Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric release has been. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented. Environmental Monitoring Pics Guidelines.
From ask.pharmaguideline.com
Environmental Monitoring surface slope Pharmaguideline Forum Environmental Monitoring Pics Guidelines In those cases where parametric release has been. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed. Environmental Monitoring Pics Guidelines.
From www.infectioncontrolresults.com
What is Healthcare Environmental Monitoring? Infection Control Results Environmental Monitoring Pics Guidelines Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. In those cases where parametric release has been. Pharmacopoeial methods. Environmental Monitoring Pics Guidelines.
From www.slideserve.com
PPT Environmental Control and Measurement PowerPoint Presentation Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Qualification, cleanroom design, process design, quality control,. Environmental Monitoring Pics Guidelines.
From www.youtube.com
Tips for an Effective Environmental Monitoring Program YouTube Environmental Monitoring Pics Guidelines Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Whereas pic/s guide pe 009 applies to industrial. Environmental Monitoring Pics Guidelines.
From www.slideserve.com
PPT Microbiological Control Tests PowerPoint Presentation, free Environmental Monitoring Pics Guidelines This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Environmental and process monitoring this. Environmental Monitoring Pics Guidelines.
From www.slideserve.com
PPT Risk and RACI Defining Clear Roles PowerPoint Presentation, free Environmental Monitoring Pics Guidelines Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Pharmacopoeial methods should be used for the validation and performance of the sterility test. Whereas pic/s guide pe 009 applies. Environmental Monitoring Pics Guidelines.
From www.scribd.com
Environmental Monitoring Management in Pharmaceutical Facilities to Environmental Monitoring Pics Guidelines Whereas pic/s guide pe 009 applies to industrial manufacture of distributed medicinal products, the basic requirements presented in. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. In those cases where parametric release has been. This guideline provides general guidance that should be used in the design and. Environmental Monitoring Pics Guidelines.
From www.digi.com
What Is Environmental Monitoring? Digi International Environmental Monitoring Pics Guidelines This guideline provides general guidance that should be used in the design and control of premises, equipment, utilities, systems and. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Pharmacopoeial methods should be used for the validation and performance of the sterility test. In those cases where parametric release has been. Environmental and process monitoring. Environmental Monitoring Pics Guidelines.
From microbiologyguideritesh.com
Environmental Monitoring 2023 Microbiology Guidelines Environmental Monitoring Pics Guidelines Pharmacopoeial methods should be used for the validation and performance of the sterility test. In those cases where parametric release has been. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid. Environmental Monitoring Pics Guidelines.
From cpawsnab.org
Alberta to resume environmental monitoring requirements for oil and gas Environmental Monitoring Pics Guidelines Environmental monitoring, or negative product controls may contribute to the verification of a false positive test finding and an invalid test. Environmental and process monitoring this section differs from guidance given in section 5 in that the guidance here. Qualification, cleanroom design, process design, quality control, environmental monitoring, and review of production records. Pharmacopoeial methods should be used for the. Environmental Monitoring Pics Guidelines.