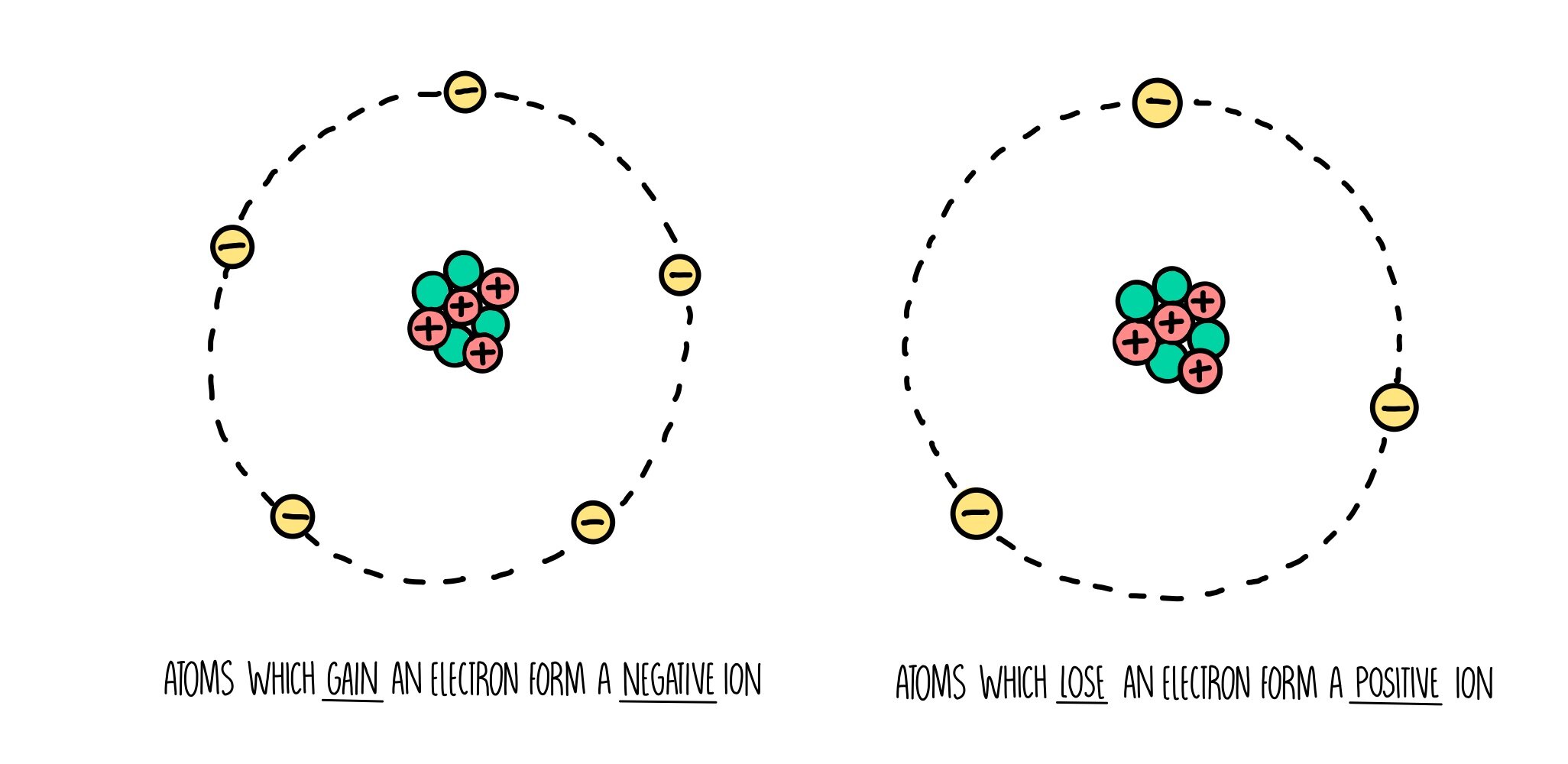
Do Ions Exist By Themselves . Most atoms do not have eight electrons in their valence electron. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Sulfur is in group 6 of the periodic table. Ions are formed by the transfer of electrons. Remember that ions are formed only when electrons move from one atom to another; What is the charge on its ions, and is the charge positive or. The charges on monatomic ions of most main group elements. A proton never moves from one atom to another. Use lewis diagrams to illustrate ion formation. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Define the two types of ions.
from www.thesciencehive.co.uk
What is the charge on its ions, and is the charge positive or. Sulfur is in group 6 of the periodic table. Use lewis diagrams to illustrate ion formation. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Most atoms do not have eight electrons in their valence electron. Remember that ions are formed only when electrons move from one atom to another; The charges on monatomic ions of most main group elements. A proton never moves from one atom to another. Ions are formed by the transfer of electrons. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5.
Ionic Bonding — the science hive
Do Ions Exist By Themselves Sulfur is in group 6 of the periodic table. Ions are formed by the transfer of electrons. Most atoms do not have eight electrons in their valence electron. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. A proton never moves from one atom to another. What is the charge on its ions, and is the charge positive or. Define the two types of ions. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Remember that ions are formed only when electrons move from one atom to another; Sulfur is in group 6 of the periodic table. The charges on monatomic ions of most main group elements. Use lewis diagrams to illustrate ion formation.
From byjus.com
Why do ions form bonds though they have already attained noble gas Do Ions Exist By Themselves Ions are formed by the transfer of electrons. Define the two types of ions. Use lewis diagrams to illustrate ion formation. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). A proton never moves from one atom to another. Most atoms do not have eight electrons in their valence electron. Remember that ions are formed only when. Do Ions Exist By Themselves.
From courses.lumenlearning.com
2.7 Molecular and Ionic Compounds Chemistry Do Ions Exist By Themselves What is the charge on its ions, and is the charge positive or. Define the two types of ions. Sulfur is in group 6 of the periodic table. Remember that ions are formed only when electrons move from one atom to another; Use lewis diagrams to illustrate ion formation. Most atoms do not have eight electrons in their valence electron.. Do Ions Exist By Themselves.
From www.reddit.com
[Chemistry] how does ion conduct electricity? Do the electrons let Do Ions Exist By Themselves During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Use lewis diagrams to illustrate ion formation. Most atoms do not have eight electrons in their valence electron. Define the two types of ions. The charges on monatomic ions of most main group elements. Ions can consist of one. Do Ions Exist By Themselves.
From www.youtube.com
What is an Ion and Examples ( in Hindi/Urdu) Chemistry Class 9 & 11 Do Ions Exist By Themselves Define the two types of ions. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Sulfur is in group 6 of the periodic table. The charges on monatomic ions of most main group elements. A proton never moves from one atom to another. Most atoms do not have. Do Ions Exist By Themselves.
From printablesalaz2j.z13.web.core.windows.net
Write The Differences Between Atoms And Ions Do Ions Exist By Themselves During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Define the two types of ions. What is the charge on its ions, and is the charge positive or. The charges on monatomic ions of most main group elements. Ions can consist of one atom (monatomic ions) or several. Do Ions Exist By Themselves.
From content.myhometuition.com
Matter and Substance user's Blog! Do Ions Exist By Themselves Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to another. Define the two types of ions. Most atoms do not have eight electrons in their valence electron. Sulfur is in group 6 of the periodic. Do Ions Exist By Themselves.
From cabinet.matttroy.net
Is Table Salt A Molecular Compound Matttroy Do Ions Exist By Themselves Ions can consist of one atom (monatomic ions) or several (polyatomic ions). The charges on monatomic ions of most main group elements. Most atoms do not have eight electrons in their valence electron. What is the charge on its ions, and is the charge positive or. Sulfur is in group 6 of the periodic table. During the formation of some. Do Ions Exist By Themselves.
From ar.inspiredpencil.com
What Is Ion Do Ions Exist By Themselves The charges on monatomic ions of most main group elements. Remember that ions are formed only when electrons move from one atom to another; What is the charge on its ions, and is the charge positive or. Use lewis diagrams to illustrate ion formation. Sulfur is in group 6 of the periodic table. Define the two types of ions. During. Do Ions Exist By Themselves.
From www.youtube.com
How are ions formed? YouTube Do Ions Exist By Themselves Sulfur is in group 6 of the periodic table. Define the two types of ions. Use lewis diagrams to illustrate ion formation. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). A proton never moves from one atom to another. Ions are formed by the transfer of electrons. Most atoms do not have eight electrons in their. Do Ions Exist By Themselves.
From wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Do Ions Exist By Themselves Sulfur is in group 6 of the periodic table. Ions are formed by the transfer of electrons. Remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to another. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Use lewis diagrams to illustrate ion formation. What. Do Ions Exist By Themselves.
From en.vidabytes.com
What power supply do I need? How do you know? Do Ions Exist By Themselves Use lewis diagrams to illustrate ion formation. Define the two types of ions. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Remember that ions are formed only when electrons move from one atom to another; Ions can consist of one atom (monatomic ions) or several (polyatomic ions).. Do Ions Exist By Themselves.
From www.youtube.com
Why Do Ions Form? YouTube Do Ions Exist By Themselves During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Most atoms do not have eight electrons in their valence electron. The charges on monatomic ions of most main group elements. A proton never moves from one atom to another. Remember that ions are formed only when electrons move. Do Ions Exist By Themselves.
From byjus.com
Why do ions form bonds though they have already attained noble gas Do Ions Exist By Themselves What is the charge on its ions, and is the charge positive or. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Define the two types of ions. Use lewis diagrams to illustrate ion formation. The charges on monatomic ions of most main group elements. Ions can consist. Do Ions Exist By Themselves.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do Ions Exist By Themselves Sulfur is in group 6 of the periodic table. Remember that ions are formed only when electrons move from one atom to another; Use lewis diagrams to illustrate ion formation. Most atoms do not have eight electrons in their valence electron. Define the two types of ions. Ions are formed by the transfer of electrons. What is the charge on. Do Ions Exist By Themselves.
From www.slideserve.com
PPT Giant Ionic Structures PowerPoint Presentation, free download Do Ions Exist By Themselves Define the two types of ions. A proton never moves from one atom to another. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Ions are formed by the transfer of electrons. The charges on monatomic ions of most main group elements. Use lewis diagrams to illustrate ion formation. Most atoms do not have eight electrons in. Do Ions Exist By Themselves.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Do Ions Exist By Themselves Sulfur is in group 6 of the periodic table. The charges on monatomic ions of most main group elements. Remember that ions are formed only when electrons move from one atom to another; A proton never moves from one atom to another. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). What is the charge on its. Do Ions Exist By Themselves.
From www.showme.com
How many ions? Chemistry, Science ShowMe Do Ions Exist By Themselves Ions are formed by the transfer of electrons. Use lewis diagrams to illustrate ion formation. Sulfur is in group 6 of the periodic table. Define the two types of ions. Most atoms do not have eight electrons in their valence electron. The charges on monatomic ions of most main group elements. A proton never moves from one atom to another.. Do Ions Exist By Themselves.
From www.youtube.com
Number of Ions in Na3PO4 Sodium phosphate YouTube Do Ions Exist By Themselves During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Define the two types of ions. The charges on monatomic ions of most main group elements. Most atoms do not have eight electrons in their valence electron.. Do Ions Exist By Themselves.
From joieueami.blob.core.windows.net
Model Of An Ion at Juanita Jenkins blog Do Ions Exist By Themselves A proton never moves from one atom to another. Ions are formed by the transfer of electrons. The charges on monatomic ions of most main group elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Ions can consist of one atom (monatomic ions) or several (polyatomic ions).. Do Ions Exist By Themselves.
From www.greelane.com
Que sont les ions spectateurs et pourquoi sontils importants Do Ions Exist By Themselves The charges on monatomic ions of most main group elements. What is the charge on its ions, and is the charge positive or. A proton never moves from one atom to another. Sulfur is in group 6 of the periodic table. Define the two types of ions. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Most. Do Ions Exist By Themselves.
From www.youtube.com
Naming Ions How to Write the Names for Ions YouTube Do Ions Exist By Themselves Define the two types of ions. Remember that ions are formed only when electrons move from one atom to another; Sulfur is in group 6 of the periodic table. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Most atoms do not have eight electrons in their valence electron. The charges on monatomic ions of most main. Do Ions Exist By Themselves.
From slideplayer.com
Polyatomic Ions. ppt download Do Ions Exist By Themselves The charges on monatomic ions of most main group elements. Remember that ions are formed only when electrons move from one atom to another; Define the two types of ions. Most atoms do not have eight electrons in their valence electron. Ions are formed by the transfer of electrons. What is the charge on its ions, and is the charge. Do Ions Exist By Themselves.
From pubs.rsc.org
Dalton perspectives. Do R3Si+ ions exist in solution? Journal of the Do Ions Exist By Themselves What is the charge on its ions, and is the charge positive or. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Define the two types of ions. Use lewis diagrams to illustrate ion formation. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). The. Do Ions Exist By Themselves.
From slideplayer.com
Ionic Charges Group 1A +1 Group 2A +2 Group 3A ppt download Do Ions Exist By Themselves Ions are formed by the transfer of electrons. Sulfur is in group 6 of the periodic table. Define the two types of ions. What is the charge on its ions, and is the charge positive or. The charges on monatomic ions of most main group elements. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Remember that. Do Ions Exist By Themselves.
From www.chemistrystudent.com
Complex Ions (ALevel) ChemistryStudent Do Ions Exist By Themselves What is the charge on its ions, and is the charge positive or. Sulfur is in group 6 of the periodic table. Ions are formed by the transfer of electrons. Most atoms do not have eight electrons in their valence electron. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). During the formation of some compounds, atoms. Do Ions Exist By Themselves.
From www.alamy.com
Classification of Ions The overall charges of an ion represent its Do Ions Exist By Themselves Define the two types of ions. What is the charge on its ions, and is the charge positive or. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Use lewis diagrams to illustrate ion formation. Remember that ions are formed only when electrons move from one atom to another; The charges on monatomic ions of most main. Do Ions Exist By Themselves.
From www.expii.com
Ionic Bond — Formation & Compounds Expii Do Ions Exist By Themselves Most atoms do not have eight electrons in their valence electron. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. What is the charge on its ions, and is the charge positive or. Use lewis diagrams to illustrate ion formation. Ions can consist of one atom (monatomic ions). Do Ions Exist By Themselves.
From www.compoundchem.com
Common Polyatomic Ions Names, Formulae, and Charges Compound Interest Do Ions Exist By Themselves What is the charge on its ions, and is the charge positive or. Define the two types of ions. Most atoms do not have eight electrons in their valence electron. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Use lewis diagrams to illustrate ion formation. Remember that ions are formed only when electrons move from one. Do Ions Exist By Themselves.
From elanra.com
IonsExplained Elanramedical Do Ions Exist By Themselves Use lewis diagrams to illustrate ion formation. Define the two types of ions. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). Most atoms do not have eight electrons in their valence electron. Ions are formed. Do Ions Exist By Themselves.
From www.youtube.com
Elements, Atoms, Molecules, Ions, Ionic and Molecular Compounds Do Ions Exist By Themselves The charges on monatomic ions of most main group elements. Use lewis diagrams to illustrate ion formation. What is the charge on its ions, and is the charge positive or. Remember that ions are formed only when electrons move from one atom to another; Define the two types of ions. Sulfur is in group 6 of the periodic table. Ions. Do Ions Exist By Themselves.
From www.pmfias.com
Molecule, Ion, Molecules of Elements & Compounds, Atomicity PMF IAS Do Ions Exist By Themselves Remember that ions are formed only when electrons move from one atom to another; Ions are formed by the transfer of electrons. A proton never moves from one atom to another. Ions can consist of one atom (monatomic ions) or several (polyatomic ions). What is the charge on its ions, and is the charge positive or. Define the two types. Do Ions Exist By Themselves.
From www.slideserve.com
PPT C9. Metal ions in biological systems PowerPoint Presentation ID Do Ions Exist By Themselves A proton never moves from one atom to another. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. Remember that ions are formed only when electrons move from one atom to another; The charges on monatomic ions of most main group elements. Sulfur is in group 6 of. Do Ions Exist By Themselves.
From techiescientist.com
HCl Intermolecular Forces — Type, Strong or Weak? Techiescientist Do Ions Exist By Themselves The charges on monatomic ions of most main group elements. Ions are formed by the transfer of electrons. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. A proton never moves from one atom to another. Sulfur is in group 6 of the periodic table. Most atoms do. Do Ions Exist By Themselves.
From socratic.org
Sodium hydroxide (NaOH) is classified as a strong base. For every mole Do Ions Exist By Themselves What is the charge on its ions, and is the charge positive or. Define the two types of ions. A proton never moves from one atom to another. Sulfur is in group 6 of the periodic table. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (figure 3.5.1 3.5. The charges. Do Ions Exist By Themselves.
From chubbyrevision-a2level.weebly.com
The Transition Metals Chubby Revision A2 Level Do Ions Exist By Themselves Sulfur is in group 6 of the periodic table. Define the two types of ions. Most atoms do not have eight electrons in their valence electron. The charges on monatomic ions of most main group elements. Use lewis diagrams to illustrate ion formation. What is the charge on its ions, and is the charge positive or. A proton never moves. Do Ions Exist By Themselves.