For Mixing Of Liquids Benzene And Toluene . Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. The vapor stream leaving at the top of the column, which contains 97 mole%. See examples of benzene and toluene, and how they deviate from ideal behavior. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. Learn how distillation works, how to use a distillation apparatus, and how. Benzene and toluene form ideal solution over the entire range of composition. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. The vapour pressure of pure benzene and toluene at 300 k are 50.71. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases.
from www.chegg.com
The vapor stream leaving at the top of the column, which contains 97 mole%. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Learn how distillation works, how to use a distillation apparatus, and how. The vapour pressure of pure benzene and toluene at 300 k are 50.71. Benzene and toluene form ideal solution over the entire range of composition. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules.
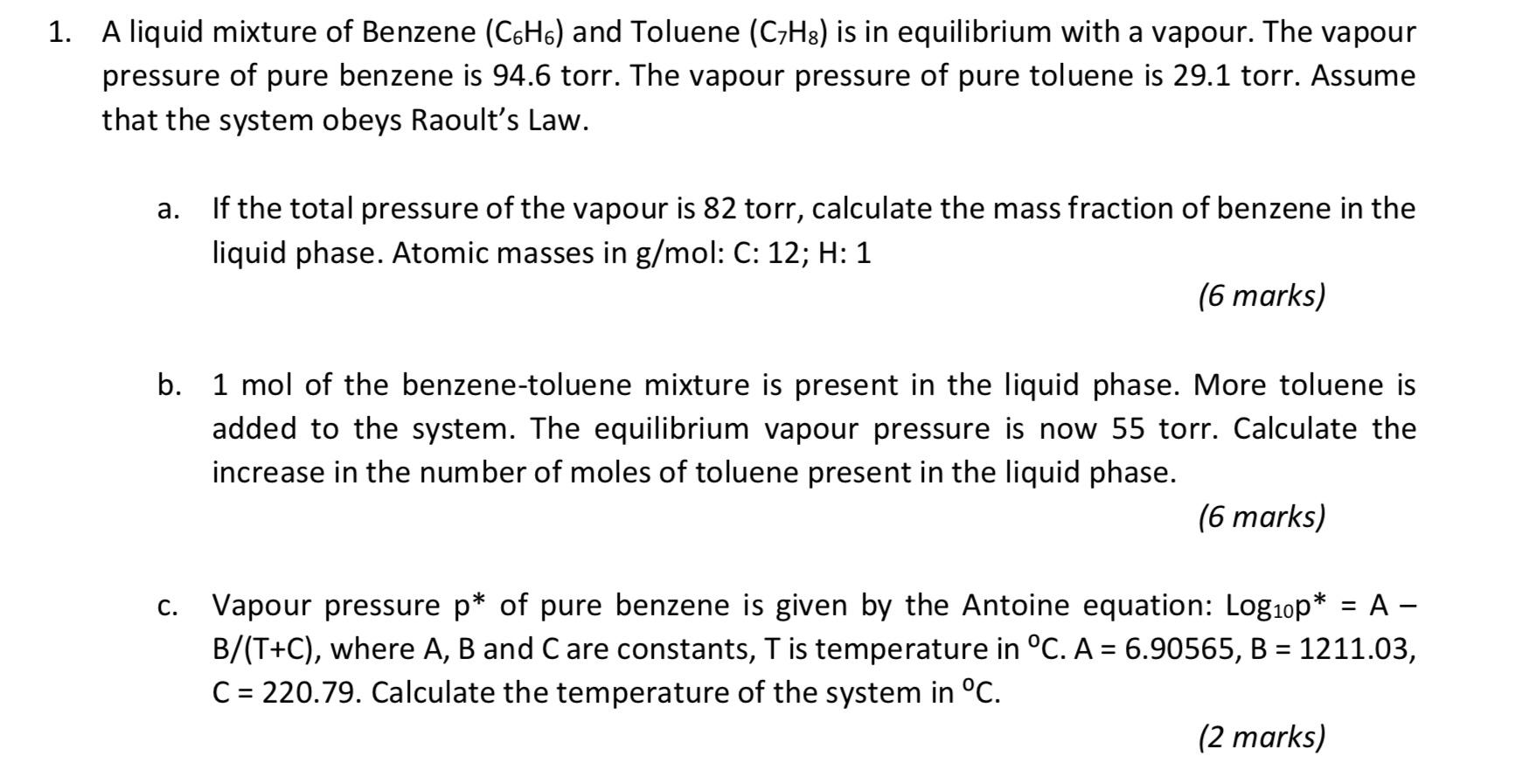
Solved 1. A liquid mixture of Benzene (C6H6) and Toluene
For Mixing Of Liquids Benzene And Toluene Benzene and toluene form ideal solution over the entire range of composition. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. See examples of benzene and toluene, and how they deviate from ideal behavior. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. The vapor stream leaving at the top of the column, which contains 97 mole%. Benzene and toluene form ideal solution over the entire range of composition. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Learn how distillation works, how to use a distillation apparatus, and how. The vapour pressure of pure benzene and toluene at 300 k are 50.71.
From www.toppr.com
Benzene and toluene form an ideal solution over the entire range of For Mixing Of Liquids Benzene And Toluene Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Benzene and toluene form ideal solution over the entire range of composition. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. The vapour pressure of pure benzene and. For Mixing Of Liquids Benzene And Toluene.
From brainly.com
An ideal liquid mixture of benzene and toluene (50 by volume each) is For Mixing Of Liquids Benzene And Toluene The vapor stream leaving at the top of the column, which contains 97 mole%. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. Learn how distillation works, how to use a. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved e) Use the following benzene/toluene Txy diagram to For Mixing Of Liquids Benzene And Toluene Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. Benzene and toluene form ideal solution over the entire range of composition. Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. See examples of benzene and toluene, and how. For Mixing Of Liquids Benzene And Toluene.
From www.bartleby.com
Answered 4.42. An equimolar liquid mixture of… bartleby For Mixing Of Liquids Benzene And Toluene Benzene and toluene form ideal solution over the entire range of composition. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. Learn how distillation works, how to use a distillation apparatus, and how.. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
SOLVED The boiling point diagram for a mixture of benzene and toluene For Mixing Of Liquids Benzene And Toluene It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. Learn how to separate a mixture of benzene and toluene using. For Mixing Of Liquids Benzene And Toluene.
From www.coursehero.com
[Solved] A liquid mixture containing 30 mole benzene (B), 25 toluene For Mixing Of Liquids Benzene And Toluene Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. See examples of benzene and toluene, and how they deviate from ideal behavior. Learn how to separate a mixture of. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved A liquid mixture of benzene (B), toluene (T) and For Mixing Of Liquids Benzene And Toluene It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Learn how distillation works, how to use a distillation apparatus, and how. The vapor. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved The Hydro Alkylation Of Toluene Is A Process Used For Mixing Of Liquids Benzene And Toluene Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. See examples of benzene and toluene, and how they deviate from ideal behavior. It is desired to separate an. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved An equimolar liquid mixture of benzene and toluene is For Mixing Of Liquids Benzene And Toluene Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. The vapor stream leaving at the top of the column, which contains 97 mole%. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol. For Mixing Of Liquids Benzene And Toluene.
From www.toppr.com
Benzene and toluene form an ideal solution. A liquid solution is formed For Mixing Of Liquids Benzene And Toluene Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Learn how distillation works, how to use a distillation apparatus, and how. Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Learn how to apply raoult's law to predict the vapor pressure. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved The separation of mixture is For Mixing Of Liquids Benzene And Toluene Learn how distillation works, how to use a distillation apparatus, and how. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Learn how to apply raoult's law to predict the vapor pressure. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved 1. A liquid mixture of Benzene (C6H6) and Toluene For Mixing Of Liquids Benzene And Toluene See examples of benzene and toluene, and how they deviate from ideal behavior. The vapor stream leaving at the top of the column, which contains 97 mole%. Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. It is desired to separate an equimolar mixture of benzene and toluene into a top. For Mixing Of Liquids Benzene And Toluene.
From www.coursehero.com
[Solved] Assume that benzene and toluene can form an ideal solution For Mixing Of Liquids Benzene And Toluene Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95. For Mixing Of Liquids Benzene And Toluene.
From www.coursehero.com
[Solved] The liquid mixture of 60 benzene and 40 toluene is charged For Mixing Of Liquids Benzene And Toluene Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. See examples of benzene and toluene, and how they deviate from ideal behavior. The vapor stream leaving at the top of the column, which. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved 35. An ideal liquid mixture of benzene and toluene For Mixing Of Liquids Benzene And Toluene Learn how distillation works, how to use a distillation apparatus, and how. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. Benzene and toluene form ideal solution over the entire range of composition. Learn how to separate a mixture. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
SOLVED Benzene and toluene form nearly ideal solutions. The boiling For Mixing Of Liquids Benzene And Toluene Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Learn how to read and use txy diagrams for binary mixtures of benzene and toluene at 1 atm. The vapor stream leaving at the top of the column, which contains 97 mole%. Benzene and toluene form ideal solution over the entire range of composition. Distillation. For Mixing Of Liquids Benzene And Toluene.
From byjus.com
Vapour pressure of pure benzene and toluene at 313K is 150 and 50 mmHg For Mixing Of Liquids Benzene And Toluene Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. Benzene and toluene form ideal solution over the entire range of composition. The vapor stream leaving at the top of the column, which contains 97 mole%. Learn how to separate a mixture of benzene and toluene using fractional. For Mixing Of Liquids Benzene And Toluene.
From cartoondealer.com
Selective Focus Of Benzene, Toluene And Xylene Liquid Chemical Compound For Mixing Of Liquids Benzene And Toluene Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Learn how distillation works, how to use a distillation apparatus, and how. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. The vapor stream leaving at the top of the column, which contains 97 mole%. Learn how. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
SOLVED Liquidvapor phase diagrams of binary solutions are drawn to For Mixing Of Liquids Benzene And Toluene Learn how distillation works, how to use a distillation apparatus, and how. The vapor stream leaving at the top of the column, which contains 97 mole%. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules.. For Mixing Of Liquids Benzene And Toluene.
From www.toppr.com
Benzene and toulene from an ideal solution, the vapour pressures of the For Mixing Of Liquids Benzene And Toluene Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Distillation is method of separation of. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
Toluene (C6H5CH3) is a liquid compound similar to benzene (C6H6 For Mixing Of Liquids Benzene And Toluene An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. See examples of benzene and. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
SOLVED 4Benzene is produced using hydrodealkylation process. During For Mixing Of Liquids Benzene And Toluene Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. The vapour pressure of pure benzene and toluene at 300 k are 50.71. Find bubble points, dew points, and mole fractions of. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved A distillation column containing a mixture of benzene For Mixing Of Liquids Benzene And Toluene See examples of benzene and toluene, and how they deviate from ideal behavior. The vapour pressure of pure benzene and toluene at 300 k are 50.71. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. An equimolar liquid mixture. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
SOLVED Refer to the vaporliquid composition curve for a benzene For Mixing Of Liquids Benzene And Toluene Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Benzene and toluene form ideal solution over the entire range of composition. The vapour pressure of pure benzene and toluene at 300 k are 50.71. The vapor stream. For Mixing Of Liquids Benzene And Toluene.
From www.toppr.com
Vapour pressure of C6H6 and C7H8 mixture at 50^∘C is given by P(mm Hg For Mixing Of Liquids Benzene And Toluene The vapor stream leaving at the top of the column, which contains 97 mole%. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Benzene and toluene form ideal solution over the entire range of composition. Distillation is method of separation of components from a liquid mixture which depends on the differences in. For Mixing Of Liquids Benzene And Toluene.
From www.dreamstime.com
Selective Focus of Benzene, Toluene and Xylene Liquid Chemical Compound For Mixing Of Liquids Benzene And Toluene Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. The vapour pressure of pure benzene and toluene at 300 k are 50.71. Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. Find bubble points, dew points, and mole fractions. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved A mixture of benzene and toluene is separated into For Mixing Of Liquids Benzene And Toluene Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a. For Mixing Of Liquids Benzene And Toluene.
From pubs.acs.org
Extractive Distillation with Ionic Liquids To Separate Benzene, Toluene For Mixing Of Liquids Benzene And Toluene Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. It is desired to separate. For Mixing Of Liquids Benzene And Toluene.
From pubs.acs.org
Extractive Distillation with Ionic Liquids To Separate Benzene, Toluene For Mixing Of Liquids Benzene And Toluene The vapor stream leaving at the top of the column, which contains 97 mole%. The vapour pressure of pure benzene and toluene at 300 k are 50.71. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. Learn how to read and use txy diagrams for binary mixtures of benzene and. For Mixing Of Liquids Benzene And Toluene.
From www.coursehero.com
[Solved] An equimolar liquid mixture of benzene and toluene is For Mixing Of Liquids Benzene And Toluene An equimolar liquid mixture of benzene and toluene is separated into two product streams by distillation. The vapor stream leaving at the top of the column, which contains 97 mole%. Learn how to apply raoult's law to predict the vapor pressure and solubility of ideal solutions of similar molecules. Find bubble points, dew points, and mole fractions of substances in. For Mixing Of Liquids Benzene And Toluene.
From brainly.in
the mole fraction of benzene in a solution with toluene is 0.5 For Mixing Of Liquids Benzene And Toluene Distillation is a technique to separate components of a liquid mixture by boiling and condensing the vapor. The vapor stream leaving at the top of the column, which contains 97 mole%. Distillation is method of separation of components from a liquid mixture which depends on the differences in boiling points of the individual. The vapour pressure of pure benzene and. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
A liquid mixture of is to be distilled in a For Mixing Of Liquids Benzene And Toluene The vapour pressure of pure benzene and toluene at 300 k are 50.71. See examples of benzene and toluene, and how they deviate from ideal behavior. The vapor stream leaving at the top of the column, which contains 97 mole%. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. Distillation is method of separation. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
SOLVED A solution is prepared by mixing 50.0 mL toluene (C6H5CH3., .d For Mixing Of Liquids Benzene And Toluene Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. Benzene and toluene form ideal solution over the entire range of composition. Find bubble points, dew points, and mole fractions of substances in liquid and vapour phases. An equimolar liquid mixture of benzene and toluene is separated into. For Mixing Of Liquids Benzene And Toluene.
From www.chegg.com
Solved 27 Phase diagram of ideal mixtures In the following For Mixing Of Liquids Benzene And Toluene Benzene and toluene form ideal solution over the entire range of composition. It is desired to separate an equimolar mixture of benzene and toluene into a top product containing 95 mol % benzene and a bottom product containing 95 mol % toluene. The vapor stream leaving at the top of the column, which contains 97 mole%. Learn how to separate. For Mixing Of Liquids Benzene And Toluene.
From www.numerade.com
An equilibrium diagram is provided that plots the mole fraction of For Mixing Of Liquids Benzene And Toluene The vapour pressure of pure benzene and toluene at 300 k are 50.71. Learn how to separate a mixture of benzene and toluene using fractional distillation, a technique for separating miscible liquids with similar boiling. The vapor stream leaving at the top of the column, which contains 97 mole%. Benzene and toluene form ideal solution over the entire range of. For Mixing Of Liquids Benzene And Toluene.