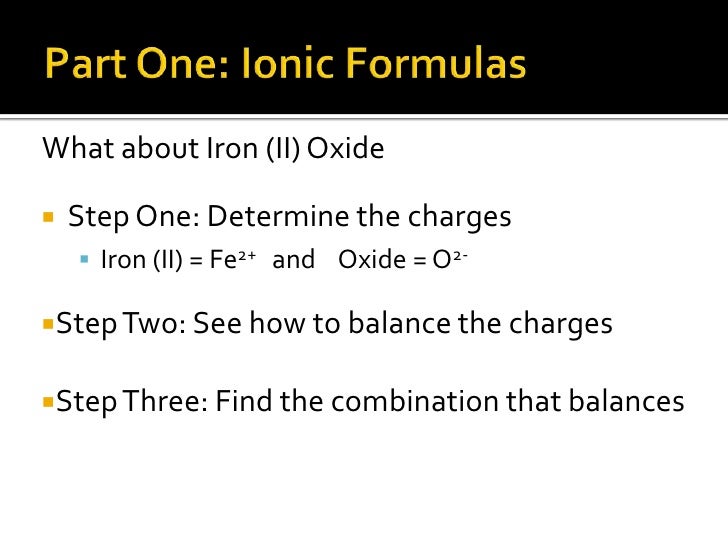
Iron(Ii) Oxide Charge . The iron(iii) ion has a formula of fe 3 +. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge; So fe +2 is iron (ii) and fe +3 is iron (iii). The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. An ionic compound is neutral, so the positive and. Some of the metals form very common ions which have latin names that are in common use, and you. The iron(ii) ion has a formula of fe 2 +.
from www.slideshare.net
Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. So fe +2 is iron (ii) and fe +3 is iron (iii). For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). An ionic compound is neutral, so the positive and. The iron(iii) ion has a formula of fe 3 +. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron( ii ) has a 2+ charge; The iron(ii) ion has a formula of fe 2 +. Some of the metals form very common ions which have latin names that are in common use, and you.
Unit 12 Chemical Naming and Formulas
Iron(Ii) Oxide Charge So fe +2 is iron (ii) and fe +3 is iron (iii). An ionic compound is neutral, so the positive and. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). Roman numeral notation indicates charge of ion when element commonly forms more than one ion. So fe +2 is iron (ii) and fe +3 is iron (iii). Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. For example, iron( ii ) has a 2+ charge; The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Some of the metals form very common ions which have latin names that are in common use, and you. The iron(iii) ion has a formula of fe 3 +. The iron(ii) ion has a formula of fe 2 +.
From www.nagwa.com
Question Video Deducing the Ionic Formula of an Ionic Compound Where Iron(Ii) Oxide Charge So fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form very common ions which have latin names that are in common use, and you. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. The formula for the iron (ii) ion is fe2+ and. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Writing Formulas and Names of Ionic Compounds PowerPoint Iron(Ii) Oxide Charge Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. The iron(ii) ion has a formula of fe 2 +. An ionic compound is neutral, so the positive and. For example, iron( ii ) has a 2+ charge; For example, knowing that iron can have a +2 or +3 charge can. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT A2 Chemistry transition metal complexes a visual guide PowerPoint Iron(Ii) Oxide Charge The iron(ii) ion has a formula of fe 2 +. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. For example, knowing that iron can have a +2 or +3 charge can. Iron(Ii) Oxide Charge.
From www.toppr.com
Iron (II) oxide crystal has a cubic structure and each edge of the unit Iron(Ii) Oxide Charge Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. An ionic compound is neutral, so the positive and. So fe +2 is iron (ii) and fe +3 is iron (iii). Some of the metals form. Iron(Ii) Oxide Charge.
From slideplayer.com
Physical Science EOC Review ppt download Iron(Ii) Oxide Charge An ionic compound is neutral, so the positive and. For example, iron( ii ) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. For example, knowing that iron can have a. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT What information do the name and formula of an ionic compound Iron(Ii) Oxide Charge The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. For example, iron( ii ) has a 2+ charge; For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). So. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download ID9452720 Iron(Ii) Oxide Charge Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. So fe +2 is iron (ii) and fe +3 is iron (iii). The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. The iron(ii) ion has a formula of fe 2 +. For. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT The Uses of Iron (II) Oxide PowerPoint Presentation, free Iron(Ii) Oxide Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). The iron(iii) ion has a formula of fe 3 +. The iron(ii) ion has a formula of fe 2 +. An ionic compound is neutral, so the positive. Iron(Ii) Oxide Charge.
From slideplayer.com
Predicting Chemical Reactions ppt download Iron(Ii) Oxide Charge The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. The iron(iii) ion has a formula of fe 3 +. So fe +2 is iron (ii) and fe +3 is iron (iii). Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Roman. Iron(Ii) Oxide Charge.
From testbook.com
Iron (II) Oxide Formula Structure, Preparation & Properties Iron(Ii) Oxide Charge The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. An ionic compound is neutral, so the positive and. The iron(iii) ion has a formula of fe 3 +. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Roman numeral notation indicates. Iron(Ii) Oxide Charge.
From www.slideshare.net
Unit 12 Chemical Naming and Formulas Iron(Ii) Oxide Charge Some of the metals form very common ions which have latin names that are in common use, and you. For example, iron( ii ) has a 2+ charge; For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide).. Iron(Ii) Oxide Charge.
From slideplayer.com
Ionic Compounds Naming ppt download Iron(Ii) Oxide Charge For example, iron( ii ) has a 2+ charge; The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. An ionic compound is neutral, so the positive and. The iron(ii) ion has a formula of fe 2 +. Because the ionic compound must be electrically neutral, it must have the same number. Iron(Ii) Oxide Charge.
From www.chegg.com
Solved The reaction between iron(II) oxide and carbon Iron(Ii) Oxide Charge For example, iron( ii ) has a 2+ charge; An ionic compound is neutral, so the positive and. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). The formula for the iron (ii) ion is fe2+ and. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Ionic Compounds Naming PowerPoint Presentation, free download Iron(Ii) Oxide Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). An ionic compound is neutral, so the positive and. The iron(iii) ion has a formula of fe 3 +. Some of the metals form very common ions which. Iron(Ii) Oxide Charge.
From www.youtube.com
How to find the Oxidation Number for Fe in FeO Iron (II) oxide YouTube Iron(Ii) Oxide Charge For example, iron( ii ) has a 2+ charge; So fe +2 is iron (ii) and fe +3 is iron (iii). For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). Because the ionic compound must be electrically. Iron(Ii) Oxide Charge.
From kunduz.com
[ANSWERED] What is the formula for iron(II) oxide ? Physical Iron(Ii) Oxide Charge So fe +2 is iron (ii) and fe +3 is iron (iii). For example, iron( ii ) has a 2+ charge; Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Some of the metals form very common ions which have latin names that are in common use, and you. An ionic compound is neutral,. Iron(Ii) Oxide Charge.
From www.youtube.com
How to Draw the Lewis Dot Structure for Fe(OH)2 Iron (II) hydroxide Iron(Ii) Oxide Charge The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. The iron(iii) ion has a formula of fe 3 +. For example, knowing that iron can have a +2 or +3 charge can. Iron(Ii) Oxide Charge.
From www.youtube.com
How to Write the Formula for Iron (II) oxide YouTube Iron(Ii) Oxide Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). Some of the metals form very common ions which have latin names that are in common use, and you. An ionic compound is neutral, so the positive and.. Iron(Ii) Oxide Charge.
From priscillanewslara.blogspot.com
Molar Mass of Iron Ii Oxide Iron(Ii) Oxide Charge The iron(ii) ion has a formula of fe 2 +. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion. Iron(Ii) Oxide Charge.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron(Ii) Oxide Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. The iron(iii) ion has a formula of fe 3 +. An ionic compound is neutral, so the positive and. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Some of the metals form very common ions. Iron(Ii) Oxide Charge.
From priscillanewslara.blogspot.com
Molar Mass of Iron Ii Oxide Iron(Ii) Oxide Charge For example, iron( ii ) has a 2+ charge; Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. An ionic compound is neutral, so the positive and. Some of the metals form very common ions. Iron(Ii) Oxide Charge.
From www.alamy.com
Iron structure Black and White Stock Photos & Images Alamy Iron(Ii) Oxide Charge For example, iron( ii ) has a 2+ charge; So fe +2 is iron (ii) and fe +3 is iron (iii). The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. The iron(iii) ion has a formula of fe 3 +. Because the ionic compound must be electrically neutral, it must have. Iron(Ii) Oxide Charge.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Iron(Ii) Oxide Charge So fe +2 is iron (ii) and fe +3 is iron (iii). The iron(ii) ion has a formula of fe 2 +. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Roman. Iron(Ii) Oxide Charge.
From www.youtube.com
How to write the equation for FeO + H2O Iron (II) oxide + Water YouTube Iron(Ii) Oxide Charge The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. So fe +2 is iron (ii) and fe +3 is iron (iii). For example, iron( ii ) has a 2+ charge; The iron(ii). Iron(Ii) Oxide Charge.
From www.pw.live
Iron II Oxide Formula Structure, Properties, Uses Iron(Ii) Oxide Charge Roman numeral notation indicates charge of ion when element commonly forms more than one ion. The iron(ii) ion has a formula of fe 2 +. The iron(iii) ion has a formula of fe 3 +. For example, iron( ii ) has a 2+ charge; An ionic compound is neutral, so the positive and. The formula for the iron (ii) ion. Iron(Ii) Oxide Charge.
From ar.inspiredpencil.com
Structural Formula Of Iron Oxide Iron(Ii) Oxide Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). So fe +2 is iron (ii) and fe +3 is iron (iii). Roman numeral notation indicates charge of ion when element commonly forms more than one ion. An. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Ionic Compounds Naming PowerPoint Presentation, free download Iron(Ii) Oxide Charge So fe +2 is iron (ii) and fe +3 is iron (iii). Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. Some of the metals form very common ions which have latin names that are in common use, and you. The formula for the iron (ii) ion is fe2+ and. Iron(Ii) Oxide Charge.
From www.youtube.com
Lewis Structure of Iron (II) Oxide, FeO YouTube Iron(Ii) Oxide Charge So fe +2 is iron (ii) and fe +3 is iron (iii). For example, iron( ii ) has a 2+ charge; The iron(iii) ion has a formula of fe 3 +. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Some of the metals form very common ions which have latin names that are. Iron(Ii) Oxide Charge.
From oneclass.com
OneClass The reaction between iron(II) oxide and carbon monoxide Iron(Ii) Oxide Charge For example, iron( ii ) has a 2+ charge; So fe +2 is iron (ii) and fe +3 is iron (iii). For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). Some of the metals form very common. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID1950987 Iron(Ii) Oxide Charge The iron(ii) ion has a formula of fe 2 +. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide).. Iron(Ii) Oxide Charge.
From www.youtube.com
How to write the formula for iron (II) oxide YouTube Iron(Ii) Oxide Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). So fe +2 is iron (ii) and fe +3 is iron (iii). For example, iron( ii ) has a 2+ charge; Because the ionic compound must be electrically. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT How to Figure Out Chemical Formulas PowerPoint Presentation, free Iron(Ii) Oxide Charge Some of the metals form very common ions which have latin names that are in common use, and you. An ionic compound is neutral, so the positive and. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. The formula for the iron (ii) ion is fe2+ and the formula for. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Writing and Naming Ionic compounds (criss cross method Iron(Ii) Oxide Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron (ii) oxide) or fe 2 o 3 (iron (iii) oxide). An ionic compound is neutral, so the positive and. Some of the metals form very common ions which have latin names that are in common use, and you.. Iron(Ii) Oxide Charge.
From www.slideserve.com
PPT Chemical Nomenclature (Naming Compounds) PowerPoint Presentation Iron(Ii) Oxide Charge The iron(ii) ion has a formula of fe 2 +. The formula for the iron (ii) ion is fe2+ and the formula for the oxide ion is o2−. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. Some of the metals form very common ions which have latin names that are in common use,. Iron(Ii) Oxide Charge.
From www.youtube.com
Iron(II) oxide YouTube Iron(Ii) Oxide Charge The iron(iii) ion has a formula of fe 3 +. Some of the metals form very common ions which have latin names that are in common use, and you. Because the ionic compound must be electrically neutral, it must have the same number of positive and negative charges. So fe +2 is iron (ii) and fe +3 is iron (iii).. Iron(Ii) Oxide Charge.