Why Solids And Liquids Are Not Included In Equilibrium . Here’s an even better explanation for why solids and liquids are excluded: A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. They are, in a way. Activities are dimensionless numbers, so a pure solid or liquid does not. Pure solids and liquids have an activity of 1. [op] why are solids and liquids not included in the equilibrium constant? Solids do not affect equilibrium as: Concentrations enter the equilibrium constant. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not.
from www.linstitute.net
They are, in a way. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Solids do not affect equilibrium as: Activities are dimensionless numbers, so a pure solid or liquid does not. A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Concentrations enter the equilibrium constant. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. Pure solids and liquids have an activity of 1. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions.
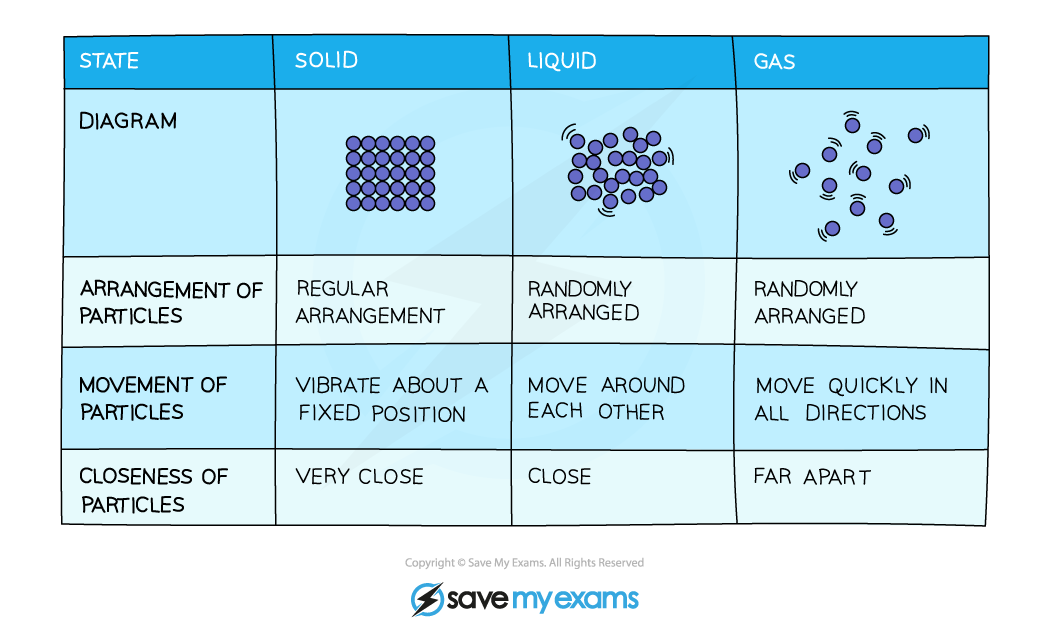
Edexcel IGCSE Chemistry 复习笔记 1.1.1 The Three States of Matter翰林国际教育
Why Solids And Liquids Are Not Included In Equilibrium Concentrations enter the equilibrium constant. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Concentrations enter the equilibrium constant. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Activities are dimensionless numbers, so a pure solid or liquid does not. A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Solids do not affect equilibrium as: Pure solids and liquids have an activity of 1. Here’s an even better explanation for why solids and liquids are excluded: They are, in a way. [op] why are solids and liquids not included in the equilibrium constant?
From joiicrblp.blob.core.windows.net
What Is Solid Liquid Solution at Nicholas Hartman blog Why Solids And Liquids Are Not Included In Equilibrium Concentrations enter the equilibrium constant. [op] why are solids and liquids not included in the equilibrium constant? Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Solids do not affect equilibrium as: They are, in a way. Pure solids and liquids have an activity of 1. Solids do not affect equilibrium as the effective. Why Solids And Liquids Are Not Included In Equilibrium.
From www.slideserve.com
PPT CHEMICAL EQUILIBRIUM PowerPoint Presentation, free download ID Why Solids And Liquids Are Not Included In Equilibrium The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. [op] why are solids and liquids not included in the equilibrium constant? Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Reactions containing pure solids and liquids results. Why Solids And Liquids Are Not Included In Equilibrium.
From studylib.net
Ch.6 L1 Solids, Liquids, Gases NOTES Why Solids And Liquids Are Not Included In Equilibrium A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Here’s an even better explanation for why solids. Why Solids And Liquids Are Not Included In Equilibrium.
From www.youtube.com
Properties of liquids How to show that liquids do not have fixed Why Solids And Liquids Are Not Included In Equilibrium Here’s an even better explanation for why solids and liquids are excluded: Solids do not affect equilibrium as: The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the. Why Solids And Liquids Are Not Included In Equilibrium.
From www.numerade.com
SOLVEDIn a final equilibrium expression solids and liquid have value Why Solids And Liquids Are Not Included In Equilibrium Here’s an even better explanation for why solids and liquids are excluded: They are, in a way. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Solids do not affect equilibrium as: A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate. Why Solids And Liquids Are Not Included In Equilibrium.
From slideplayer.com
Equilibrium. ppt download Why Solids And Liquids Are Not Included In Equilibrium Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. Solids do not affect equilibrium as: [op] why are solids and liquids not included in the equilibrium constant? Solids do not affect equilibrium as. Why Solids And Liquids Are Not Included In Equilibrium.
From www.slideshare.net
Ap chem unit 13 presentation Why Solids And Liquids Are Not Included In Equilibrium The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids. Why Solids And Liquids Are Not Included In Equilibrium.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as: Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Here’s an even better explanation for why solids and liquids are excluded: A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in. Why Solids And Liquids Are Not Included In Equilibrium.
From exycfidyi.blob.core.windows.net
Density Of Solid Liquid And Gas Class 9 at Megan Shearer blog Why Solids And Liquids Are Not Included In Equilibrium A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. They are, in a way. [op] why are solids and liquids not included in the equilibrium constant? Solids do. Why Solids And Liquids Are Not Included In Equilibrium.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Solids And Liquids Are Not Included In Equilibrium A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Solids do not affect equilibrium as: [op] why are solids and liquids not included in the equilibrium constant? Concentrations enter the equilibrium constant. The concentrations of pure solids, pure liquids, and solvents are omitted. Why Solids And Liquids Are Not Included In Equilibrium.
From www.youtube.com
Pure solids & Pure liquids can be ignored while writing expression for Why Solids And Liquids Are Not Included In Equilibrium Activities are dimensionless numbers, so a pure solid or liquid does not. Concentrations enter the equilibrium constant. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. Solids do not affect equilibrium as: A solid or liquid that should take part in a solution or gas phase reaction has. Why Solids And Liquids Are Not Included In Equilibrium.
From www.solnpharma.com
Difference Between Solid and Liquid Mixing Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Concentrations enter the equilibrium constant. Here’s an even better explanation for why solids and liquids are excluded: A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any.. Why Solids And Liquids Are Not Included In Equilibrium.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.1.1 The Three States of Matter翰林国际教育 Why Solids And Liquids Are Not Included In Equilibrium Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. [op] why are solids and liquids not included in the equilibrium constant? Activities are dimensionless numbers, so a pure solid or liquid does not. The concentrations of pure. Why Solids And Liquids Are Not Included In Equilibrium.
From studylib.net
Equilibrium for a general reaction Why Solids And Liquids Are Not Included In Equilibrium Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Here’s an even better explanation for why solids and liquids are excluded: Solids. Why Solids And Liquids Are Not Included In Equilibrium.
From blog-0734275gh.blogspot.com
28+ nett Bild Definition Of Solid Matter Define Matter In Science Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. Activities are dimensionless numbers, so a pure solid or liquid does not. Concentrations enter the equilibrium constant. [op] why are solids and. Why Solids And Liquids Are Not Included In Equilibrium.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation Why Solids And Liquids Are Not Included In Equilibrium The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Pure solids and liquids have an activity of 1. They are, in a way. Solids do not affect equilibrium as: Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous. Why Solids And Liquids Are Not Included In Equilibrium.
From dxojeueds.blob.core.windows.net
Solids Liquids And Gases Third Grade at Ann Trotter blog Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. [op] why are solids and liquids not included in the equilibrium constant? Pure solids and liquids have an activity of 1. Solids do not affect equilibrium as: Activities are dimensionless numbers, so a pure solid or liquid does not. Learn how different phases. Why Solids And Liquids Are Not Included In Equilibrium.
From www.youtube.com
15.5 Heterogeneous Equilibrium Equilibrium Expression Involving a Why Solids And Liquids Are Not Included In Equilibrium The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as: Here’s an even better explanation for why solids and liquids are excluded: They are, in a way. Reactions containing pure solids and liquids results in heterogeneous reactions in. Why Solids And Liquids Are Not Included In Equilibrium.
From byjus.com
Activity To show that solids and liquids cannot be compressed but gases Why Solids And Liquids Are Not Included In Equilibrium Concentrations enter the equilibrium constant. [op] why are solids and liquids not included in the equilibrium constant? Pure solids and liquids have an activity of 1. Solids do not affect equilibrium as: They are, in a way. Activities are dimensionless numbers, so a pure solid or liquid does not. Reactions containing pure solids and liquids results in heterogeneous reactions in. Why Solids And Liquids Are Not Included In Equilibrium.
From stock.adobe.com
states of matter solids liquids and gases. Matter appears in three Why Solids And Liquids Are Not Included In Equilibrium They are, in a way. Concentrations enter the equilibrium constant. Pure solids and liquids have an activity of 1. [op] why are solids and liquids not included in the equilibrium constant? Here’s an even better explanation for why solids and liquids are excluded: Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete.. Why Solids And Liquids Are Not Included In Equilibrium.
From www.chegg.com
Solved I. Which phases are not included in equilibrium Why Solids And Liquids Are Not Included In Equilibrium Concentrations enter the equilibrium constant. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Activities are dimensionless numbers, so a pure solid or liquid does not. Pure solids and liquids have an activity of 1. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Here’s an. Why Solids And Liquids Are Not Included In Equilibrium.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Why Solids And Liquids Are Not Included In Equilibrium [op] why are solids and liquids not included in the equilibrium constant? Solids do not affect equilibrium as: Activities are dimensionless numbers, so a pure solid or liquid does not. A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Here’s an even better. Why Solids And Liquids Are Not Included In Equilibrium.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Solids And Liquids Are Not Included In Equilibrium [op] why are solids and liquids not included in the equilibrium constant? Solids do not affect equilibrium as: Activities are dimensionless numbers, so a pure solid or liquid does not. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Concentrations enter the equilibrium constant. Solids do not affect equilibrium as the effective concentration of. Why Solids And Liquids Are Not Included In Equilibrium.
From www.youtube.com
SolidLiquid Chemical Equilibrium YouTube Why Solids And Liquids Are Not Included In Equilibrium Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as the effective concentration of it remains the. Why Solids And Liquids Are Not Included In Equilibrium.
From courses.lumenlearning.com
8.2 Solids and Liquids The Basics of General, Organic, and Biological Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Here’s an even better explanation for why solids and liquids are excluded: Solids do not affect equilibrium. Why Solids And Liquids Are Not Included In Equilibrium.
From www.numerade.com
SOLVED When writing the expression for an equilibrium constant, which Why Solids And Liquids Are Not Included In Equilibrium Pure solids and liquids have an activity of 1. Activities are dimensionless numbers, so a pure solid or liquid does not. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. [op] why are solids and liquids not included in the equilibrium constant? They. Why Solids And Liquids Are Not Included In Equilibrium.
From joihurnut.blob.core.windows.net
Why Does The Liquid Overflow From These Cups at Kevin Kugler blog Why Solids And Liquids Are Not Included In Equilibrium The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Here’s an even better explanation for why solids and liquids are excluded: Activities are dimensionless numbers, so a pure. Why Solids And Liquids Are Not Included In Equilibrium.
From www.numerade.com
SOLVEDWhy are the concentrations of pure liquids and solids not Why Solids And Liquids Are Not Included In Equilibrium Activities are dimensionless numbers, so a pure solid or liquid does not. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as: Pure solids and liquids have an activity of 1. A solid or liquid that should take. Why Solids And Liquids Are Not Included In Equilibrium.
From sciencetallis.weebly.com
3. Particle Model of Matter THOMAS TALLIS SCIENCE Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as: Here’s an even better explanation for why solids and liquids are excluded: [op] why are solids and liquids not included in the equilibrium constant? A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Pure solids and liquids. Why Solids And Liquids Are Not Included In Equilibrium.
From www.pinterest.com
Pin on School Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Solids do not affect equilibrium as: Here’s an even better explanation for why solids and liquids are excluded: Concentrations enter the equilibrium constant. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are. Why Solids And Liquids Are Not Included In Equilibrium.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Why Solids And Liquids Are Not Included In Equilibrium [op] why are solids and liquids not included in the equilibrium constant? Here’s an even better explanation for why solids and liquids are excluded: Concentrations enter the equilibrium constant. Pure solids and liquids have an activity of 1. Activities are dimensionless numbers, so a pure solid or liquid does not. A solid or liquid that should take part in a. Why Solids And Liquids Are Not Included In Equilibrium.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement Why Solids And Liquids Are Not Included In Equilibrium The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. Activities are dimensionless numbers, so a pure solid or liquid does not. Here’s an even better explanation. Why Solids And Liquids Are Not Included In Equilibrium.
From igcsechemistryrevision.weebly.com
1.1 Understand the arrangement, movement and energy of particles in Why Solids And Liquids Are Not Included In Equilibrium Concentrations enter the equilibrium constant. Learn how different phases affect equilibria and how to calculate equilibrium constants for heterogeneous reactions. Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. Solids do not affect equilibrium as: A solid or liquid that should take part in a solution or gas. Why Solids And Liquids Are Not Included In Equilibrium.
From exyfeersx.blob.core.windows.net
Why Solid Liquid And Gas Have Different Properties at Helen Gatlin blog Why Solids And Liquids Are Not Included In Equilibrium Reactions containing pure solids and liquids results in heterogeneous reactions in which the concentrations of the solids and liquids are not. The concentrations of pure solids, pure liquids, and solvents are omitted from equilibrium constant expressions because they do not change significantly during reactions when enough is. Solids do not affect equilibrium as: Concentrations enter the equilibrium constant. Solids do. Why Solids And Liquids Are Not Included In Equilibrium.
From www.pinterest.com.au
Liquid Definition Examples of Liquids Solid liquid gas, States of Why Solids And Liquids Are Not Included In Equilibrium Solids do not affect equilibrium as the effective concentration of it remains the same throughout the complete. A solid or liquid that should take part in a solution or gas phase reaction has to dissolve or evaporate before it can react in any. Pure solids and liquids have an activity of 1. Here’s an even better explanation for why solids. Why Solids And Liquids Are Not Included In Equilibrium.