Medical Device Complaint Definition . Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Complaint handling is an integral part of medical device companies’ quality management system (qms).
from www.simplerqms.com
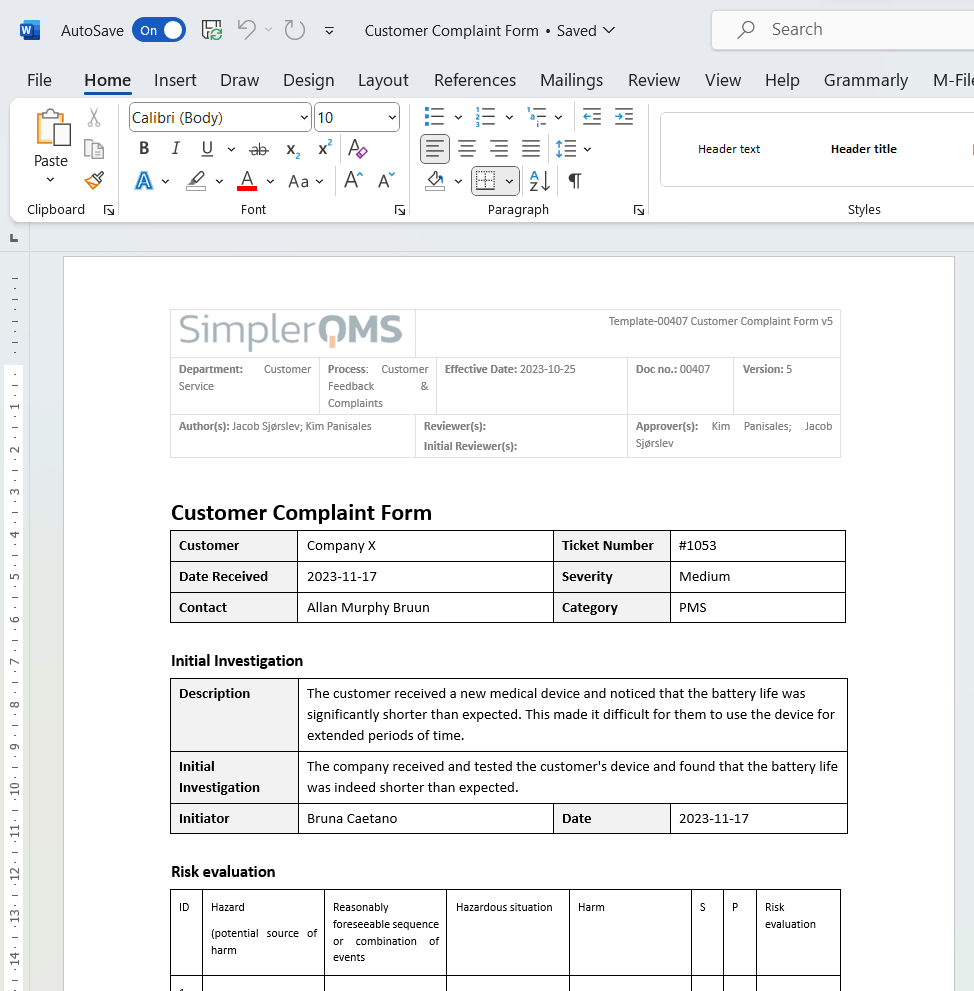
The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Complaint handling is an integral part of medical device companies’ quality management system (qms). On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda.
Medical Device Complaint Handling Process
Medical Device Complaint Definition The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Yet, complaint handling in the device industry involves so much more than placating upset customers. Complaint handling is an integral part of medical device companies’ quality management system (qms). On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following.
From rocprivateclinic.com
Complaints ROC Private Clinic Medical Device Complaint Definition Complaint handling is an integral part of medical device companies’ quality management system (qms). The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to be an iso. Medical Device Complaint Definition.
From www.greenlight.guru
Medical Device Complaint Handling Procedure [+Flowchart] Medical Device Complaint Definition Complaint handling is an integral part of medical device companies’ quality management system (qms). Yet, complaint handling in the device industry involves so much more than placating upset customers. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. No matter the. Medical Device Complaint Definition.
From www.youtube.com
Secrets of Medical Device Complaint Management Short YouTube Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21. Medical Device Complaint Definition.
From www.pdffiller.com
Fillable Online MEDICAL DEVICE COMPLAINT/ INCIDENT FORM Fax Email Print Medical Device Complaint Definition Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. On the other hand, iso 13485:2016 specifies the scope of a complaint. Medical Device Complaint Definition.
From www.qualitymeddev.com
Complaint Handling Process for Medical Device Manufacturers Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. The medical device complaint handling process is a systematic approach. Medical Device Complaint Definition.
From www.greenlight.guru
Medical Device Complaint Handling Flow Chart [Free Download] Medical Device Complaint Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Complaint handling is an integral. Medical Device Complaint Definition.
From www.defectmaestro.com
Defect Maestro AI for medical device complaints Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Complaint handling is an integral part of medical device companies’ quality management system (qms). On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges. Medical Device Complaint Definition.
From issuu.com
Medical device complaint handling by John Medical Device Complaint Definition Complaint handling is an integral part of medical device companies’ quality management system (qms). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating. Medical Device Complaint Definition.
From www.greenlight.guru
Free Complaint Template for Medical Devices Medical Device Complaint Definition The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers,. Medical Device Complaint Definition.
From medicaldevicehq.com
A guide to quality management for medical devices and ISO 13485 Medical Device Complaint Definition The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. Complaint handling is an integral part of medical device companies’ quality management system (qms). Yet, complaint. Medical Device Complaint Definition.
From www.youtube.com
Complaint Handling in Compliance with FDA and ISO Regulations YouTube Medical Device Complaint Definition Complaint handling is an integral part of medical device companies’ quality management system (qms). The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. Yet,. Medical Device Complaint Definition.
From www.youtube.com
Medical Device Complaint Handling MDR, Reports of Removals and Medical Device Complaint Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Complaint handling is an integral part of medical device companies’ quality management system (qms). On the other hand, iso 13485:2016 specifies the scope of a complaint by. Medical Device Complaint Definition.
From blog.medfriendly.com
MedFriendly Medical Blog What happens when a patient files a medical Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Complaint handling is an integral part of medical device companies’ quality management system (qms). The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr. Medical Device Complaint Definition.
From aplyonqms.com
Medical Device Complaint Trending Procedure A. P. LYON Medical Device Complaint Definition Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device reporting (mdr) regulation (21. Medical Device Complaint Definition.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint Medical Device Complaint Definition On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Complaint handling is an integral part of medical device companies’ quality management system (qms). Yet,. Medical Device Complaint Definition.
From www.linkedin.com
5 Essential Steps for FDA Complaint Handling Medical Device Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. The medical device complaint handling process is a systematic approach. Medical Device Complaint Definition.
From exojxchib.blob.core.windows.net
How To Make A Formal Medical Complaint at Janice Payne blog Medical Device Complaint Definition Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Complaint handling is an integral part of medical device companies’ quality management. Medical Device Complaint Definition.
From www.youtube.com
Medical Device Complaint Handling Systems YouTube Medical Device Complaint Definition Complaint handling is an integral part of medical device companies’ quality management system (qms). No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Yet, complaint handling in the device industry. Medical Device Complaint Definition.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint Medical Device Complaint Definition The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. No matter the risk level of your medical device, in order to be an iso 13485. Medical Device Complaint Definition.
From www.slideserve.com
PPT Customer Complaint Handling Process for Medical Device PowerPoint Medical Device Complaint Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to. Medical Device Complaint Definition.
From www.powershow.com
PPT Medical Device Complaint Management PowerPoint presentation Medical Device Complaint Definition On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device reporting (mdr) regulation (21 cfr part 803). Medical Device Complaint Definition.
From www.linkedin.com
How to Address Complaints with the Medical Device Industry Medical Device Complaint Definition The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Yet, complaint handling in the. Medical Device Complaint Definition.
From www.slideserve.com
PPT Quality System Regulation IMDMC FDA Regulatory 101 August 19 Medical Device Complaint Definition The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Complaint handling is an integral part of medical device companies’ quality management system (qms). Yet, complaint handling in the device industry involves. Medical Device Complaint Definition.
From www.greenlight.guru
How To Reduce & Prevent Medical Device Complaints Medical Device Complaint Definition Yet, complaint handling in the device industry involves so much more than placating upset customers. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. Complaint handling is an integral part of medical device companies’ quality management system (qms). No matter the. Medical Device Complaint Definition.
From www.scribd.com
FDA Medical Device Complaint Form PDF Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Complaint handling is an integral. Medical Device Complaint Definition.
From www.simplerqms.com
Medical Device Complaint Handling Process Medical Device Complaint Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to. Medical Device Complaint Definition.
From www.aplyon.com
Complaint Handling Procedure Bundle Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. The medical device complaint handling process is a systematic approach. Medical Device Complaint Definition.
From medicaldeviceacademy.com
SYS018 complaint handling procedure Medical Device Academy Medical Device Complaint Definition On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and.. Medical Device Complaint Definition.
From www.simplerqms.com
Medical Device Complaint Handling Process Medical Device Complaint Definition Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that. Medical Device Complaint Definition.
From issuu.com
Medical Device Complaint Handling Training by Medical Device GMP Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. On the other hand, iso 13485:2016. Medical Device Complaint Definition.
From chartexamples.com
Medical Device Complaint Handling Flowchart Chart Examples Medical Device Complaint Definition The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. Yet, complaint handling in the device industry involves so much more than placating upset customers. No matter the risk level of your medical device, in order to. Medical Device Complaint Definition.
From www.slideserve.com
PPT Medical Device Complaint Management Market PowerPoint Medical Device Complaint Definition Yet, complaint handling in the device industry involves so much more than placating upset customers. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. Complaint handling is an integral part of medical device companies’ quality management system (qms). On the other hand, iso 13485:2016 specifies the scope of a complaint by defining. Medical Device Complaint Definition.
From www.slideserve.com
PPT Medical Device Complaint Management Market Strategy Forecast till Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral. Medical Device Complaint Definition.
From medicaldeviceacademy.com
SYS018 complaint handling procedure Medical Device Academy Medical Device Complaint Definition Complaint handling is an integral part of medical device companies’ quality management system (qms). The medical device complaint handling process is a systematic approach employed by companies to efficiently manage. The medical device reporting (mdr) regulation (21 cfr part 803) contains mandatory requirements for manufacturers, importers, and. No matter the risk level of your medical device, in order to be. Medical Device Complaint Definition.
From www.defectmaestro.com
5 Steps to Streamline Your Medical Device Complaint Handling Process Medical Device Complaint Definition No matter the risk level of your medical device, in order to be an iso 13485 certified company and comply with fda. On the other hand, iso 13485:2016 specifies the scope of a complaint by defining it as a “written, electronic, or oral communication that alleges deficiencies” in the following. Complaint handling is an integral part of medical device companies’. Medical Device Complaint Definition.