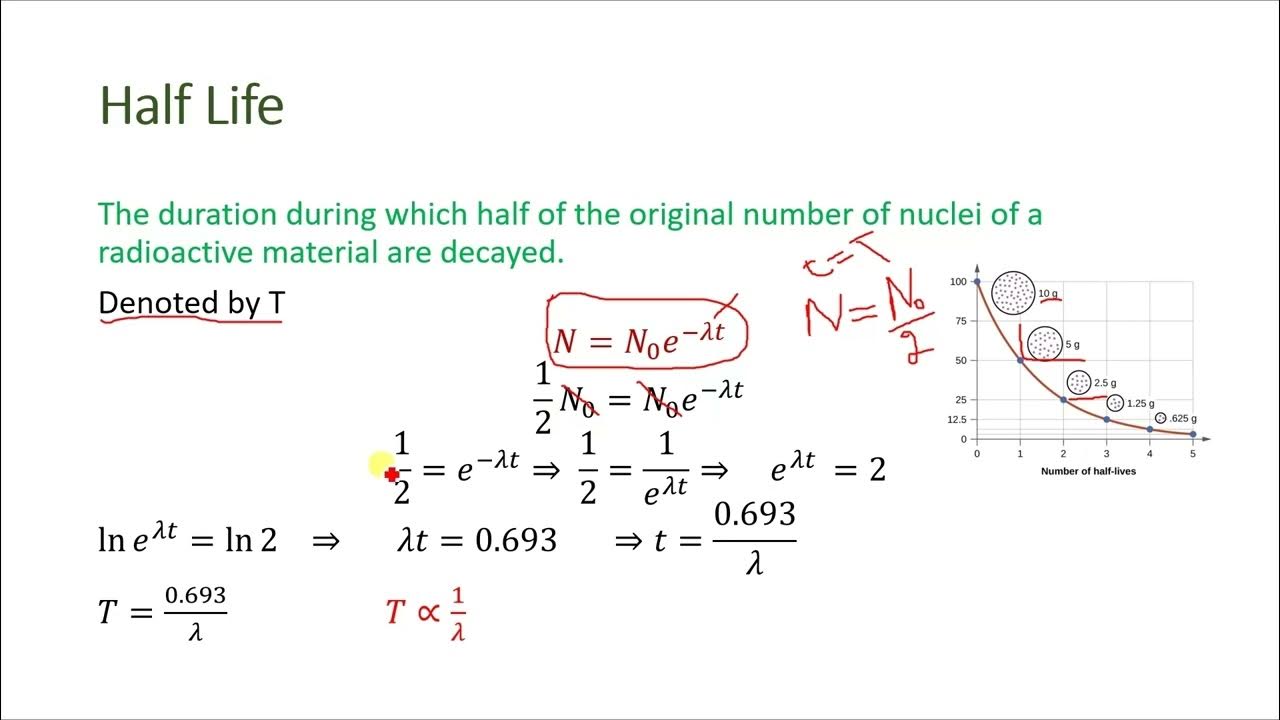
Half Life Equation T 1 2 . For example, if the initial concentration of a reactant is. We need to isolate t1 / 2 when. For a first order reaction a products , rate = k [a]: [a] = [a]oe − kt. T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: For a second order reaction 2a products or a. In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. The following equation is generated by integrating these values into. If two reactions have the same order, the T ½ = 0.693 / k.
from www.youtube.com
[a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: If two reactions have the same order, the [a] = [a]oe − kt. The following equation is generated by integrating these values into. For a zero order reaction a products , rate = k: For example, if the initial concentration of a reactant is. We need to isolate t1 / 2 when.
Half life equation derivation Radioactivity YouTube
Half Life Equation T 1 2 T ½ = 0.693 / k. The following equation is generated by integrating these values into. For example, if the initial concentration of a reactant is. In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: If two reactions have the same order, the T ½ = 0.693 / k. [a] = [a]oe − kt. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: We need to isolate t1 / 2 when. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. For a zero order reaction a products , rate = k: For a second order reaction 2a products or a.
From www.slideserve.com
PPT chapter 3 PowerPoint Presentation, free download ID3032902 Half Life Equation T 1 2 For a zero order reaction a products , rate = k: T ½ = 0.693 / k. For a second order reaction 2a products or a. The following equation is generated by integrating these values into. T ½ = [a o] / 2k. [a] = [a]oe − kt. In order to solve the half life of first order reactions, we. Half Life Equation T 1 2.
From yoursoundboard.blogspot.com
half life formula for first order reaction Ashlee Manley Half Life Equation T 1 2 For a zero order reaction a products , rate = k: For example, if the initial concentration of a reactant is. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1. Half Life Equation T 1 2.
From www.youtube.com
Half Life Equation Derivation YouTube Half Life Equation T 1 2 T ½ = 0.693 / k. For example, if the initial concentration of a reactant is. For a second order reaction 2a products or a. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. [a] = [a]oe. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 T ½ = 0.693 / k. For example, if the initial concentration of a reactant is. We need to isolate t1 / 2 when. For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k. The following equation is generated by integrating these values into. For a second order reaction 2a products. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife Equation For Firstorder Reactions T1/2... Half Life Equation T 1 2 For a zero order reaction a products , rate = k: The following equation is generated by integrating these values into. For a second order reaction 2a products or a. T ½ = [a o] / 2k. T ½ = 0.693 / k. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions t_1/2 Half Life Equation T 1 2 For a first order reaction a products , rate = k [a]: For a second order reaction 2a products or a. T ½ = 0.693 / k. The following equation is generated by integrating these values into. [a] = [a]oe − kt. T ½ = [a o] / 2k. We need to isolate t1 / 2 when. If two reactions. Half Life Equation T 1 2.
From www.youtube.com
Understanding Halflife formulas with example. YouTube Half Life Equation T 1 2 For a zero order reaction a products , rate = k: The following equation is generated by integrating these values into. For a second order reaction 2a products or a. For example, if the initial concentration of a reactant is. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = −. Half Life Equation T 1 2.
From www.numerade.com
SOLVED Halflife equation for firstorder reactions t1/2 = 0.693/k where t1/2 is the halflife Half Life Equation T 1 2 T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]: [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. For a zero order reaction a products , rate =. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions t1/2 Half Life Equation T 1 2 In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: T ½ = 0.693 / k. The following equation is generated by integrating these values into. For example, if the initial concentration of a reactant is. For a first order reaction a products , rate = k. Half Life Equation T 1 2.
From chemistnotes.com
Half life Formula Definition, and Wellderived equation Chemistry Notes Half Life Equation T 1 2 For a zero order reaction a products , rate = k: In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: [a] = [a]oe − kt. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions 0.693 Half Life Equation T 1 2 The following equation is generated by integrating these values into. T ½ = [a o] / 2k. For example, if the initial concentration of a reactant is. If two reactions have the same order, the [a] = [a]oe − kt. For a second order reaction 2a products or a. In order to solve the half life of first order reactions,. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder Half Life Equation T 1 2 T ½ = [a o] / 2k. For a zero order reaction a products , rate = k: For example, if the initial concentration of a reactant is. If two reactions have the same order, the For a second order reaction 2a products or a. [a] = [a]oe − kt. The following equation is generated by integrating these values into.. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions The Half Life Equation T 1 2 [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. We need to isolate t1 / 2 when. T ½ = [a o] / 2k. In order to solve the half life of first order reactions, we recall. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 For example, if the initial concentration of a reactant is. T ½ = [a o] / 2k. The following equation is generated by integrating these values into. [a] = [a]oe − kt. If two reactions have the same order, the For a first order reaction a products , rate = k [a]: For a second order reaction 2a products or. Half Life Equation T 1 2.
From studylib.net
HALFLIFE EQUATIONS Half Life Equation T 1 2 For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. For a second order reaction 2a products or a. The following equation is generated by integrating these values into. For example, if the initial concentration of a reactant is. [a]0 2 = [a]oe − kt1 / 2 1 2 = e −. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 If two reactions have the same order, the For a zero order reaction a products , rate = k: T ½ = [a o] / 2k. For example, if the initial concentration of a reactant is. For a second order reaction 2a products or a. The following equation is generated by integrating these values into. In order to solve the. Half Life Equation T 1 2.
From www.chegg.com
Solved (a) Demonstrate that the halflife (t1/2) of a Half Life Equation T 1 2 [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. If two reactions have the same order, the T ½ = [a o] / 2k. In order to solve the half life of first order reactions, we recall. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 If two reactions have the same order, the T ½ = 0.693 / k. The following equation is generated by integrating these values into. For a second order reaction 2a products or a. We need to isolate t1 / 2 when. [a] = [a]oe − kt. T ½ = [a o] / 2k. In order to solve the half life. Half Life Equation T 1 2.
From www.slideserve.com
PPT Chapter 14 aka Rates of Reactions PowerPoint Presentation ID3828118 Half Life Equation T 1 2 For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. We need to isolate t1 / 2 when. If two reactions have the same order, the The following equation is generated by integrating these values into. T ½ = [a o] / 2k. In order to solve the half life of first. Half Life Equation T 1 2.
From www.youtube.com
C7 HalfLife Calculations (N=Noelamda t) (t1/2=ln2/lamda) [HL IB Chemistry] YouTube Half Life Equation T 1 2 [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. For a first order reaction a products , rate = k [a]: T ½ = 0.693 / k. For a second order reaction 2a products or a. [a]. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife Equation For Firstorder Reactions T1/2... Half Life Equation T 1 2 T ½ = 0.693 / k. For a second order reaction 2a products or a. [a] = [a]oe − kt. If two reactions have the same order, the For a zero order reaction a products , rate = k: For a first order reaction a products , rate = k [a]: For example, if the initial concentration of a reactant. Half Life Equation T 1 2.
From www.wikihow.com
How to Calculate Half Life 6 Steps (with Pictures) wikiHow Half Life Equation T 1 2 The following equation is generated by integrating these values into. For a first order reaction a products , rate = k [a]: We need to isolate t1 / 2 when. For a second order reaction 2a products or a. [a] = [a]oe − kt. If two reactions have the same order, the In order to solve the half life of. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 For a zero order reaction a products , rate = k: [a] = [a]oe − kt. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. T ½ = 0.693 / k. T ½ = [a o] /. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 If two reactions have the same order, the The following equation is generated by integrating these values into. For a second order reaction 2a products or a. T ½ = 0.693 / k. We need to isolate t1 / 2 when. For a first order reaction a products , rate = k [a]: T ½ = [a o] / 2k.. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions t/2 = Half Life Equation T 1 2 We need to isolate t1 / 2 when. For a zero order reaction a products , rate = k: For a first order reaction a products , rate = k [a]: In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: T ½ = [a o] /. Half Life Equation T 1 2.
From www.slideserve.com
PPT Activities and HalfLives PowerPoint Presentation, free download ID3877683 Half Life Equation T 1 2 For a zero order reaction a products , rate = k: T ½ = 0.693 / k. For a first order reaction a products , rate = k [a]: We need to isolate t1 / 2 when. T ½ = [a o] / 2k. In order to solve the half life of first order reactions, we recall that the rate. Half Life Equation T 1 2.
From www.youtube.com
Half life equation derivation Radioactivity YouTube Half Life Equation T 1 2 In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: We need to isolate t1 / 2 when. For example, if the initial concentration of a reactant is.. Half Life Equation T 1 2.
From www.numerade.com
SOLVED Halflife equation for firstorder reactions t 1/2 = 0.693 k where t 1/2 is the half Half Life Equation T 1 2 The following equation is generated by integrating these values into. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k.. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 For a second order reaction 2a products or a. The following equation is generated by integrating these values into. In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: T ½ = [a o] / 2k. If two reactions have the same order, the T ½ =. Half Life Equation T 1 2.
From www.slideserve.com
PPT Concepts of Pharmacology Half Life Calculation PowerPoint Presentation ID307737 Half Life Equation T 1 2 For example, if the initial concentration of a reactant is. For a zero order reaction a products , rate = k: T ½ = 0.693 / k. For a second order reaction 2a products or a. [a] = [a]oe − kt. The following equation is generated by integrating these values into. For a first order reaction a products , rate. Half Life Equation T 1 2.
From www.slideserve.com
PPT Chemistry 102(01) Spring 2012 PowerPoint Presentation, free download ID6091559 Half Life Equation T 1 2 For a zero order reaction a products , rate = k: The following equation is generated by integrating these values into. For a first order reaction a products , rate = k [a]: In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: [a]0 2 = [a]oe. Half Life Equation T 1 2.
From www.chegg.com
Solved Part C PLEASE HELP Halflife equation for Half Life Equation T 1 2 [a] = [a]oe − kt. T ½ = 0.693 / k. For a second order reaction 2a products or a. We need to isolate t1 / 2 when. In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was: For a first order reaction a products , rate. Half Life Equation T 1 2.
From warreninstitute.org
Master The HALFLIFE Equation Derivation Dive Deeper! Half Life Equation T 1 2 The following equation is generated by integrating these values into. T ½ = [a o] / 2k. For a first order reaction a products , rate = k [a]: If two reactions have the same order, the In order to solve the half life of first order reactions, we recall that the rate law of a first order reaction was:. Half Life Equation T 1 2.
From www.chegg.com
Solved Halflife equation for firstorder reactions Half Life Equation T 1 2 For a second order reaction 2a products or a. For a first order reaction a products , rate = k [a]: The following equation is generated by integrating these values into. For example, if the initial concentration of a reactant is. T ½ = 0.693 / k. [a] = [a]oe − kt. We need to isolate t1 / 2 when.. Half Life Equation T 1 2.
From www.inchcalculator.com
HalfLife Calculator Inch Calculator Half Life Equation T 1 2 For example, if the initial concentration of a reactant is. [a]0 2 = [a]oe − kt1 / 2 1 2 = e − kt1 / 2 ln1 2 = − kt1 / 2 t1 / 2 = ln2 k ≈ 0.693 k. For a zero order reaction a products , rate = k: T ½ = [a o] / 2k.. Half Life Equation T 1 2.