How To Find The Product Of A Combustion Reaction . Combustion is a reaction in which a substance reacts with oxygen gas. C 2 h 5 oh (l) + o 2 (g) →. What is formed in any combustion reaction? Here is a brief overview of the solution steps before doing some examples: Read through the given information in the problem for the chemical reaction. What is needed for a combustion reaction to take place? Identify the reactants and products. (1) identify the reaction as combustion: In general chemistry, it is one of the main. Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. How to figure out the right (or product side): Since c 2 h 5 oh contains carbon, co. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. (2) know that the combustion products.
from worksheetlistfro.z13.web.core.windows.net
1) determine the grams of each element present in the original. What is formed in any combustion reaction? What is needed for a combustion reaction to take place? Identify the reactants and products. C 2 h 5 oh (l) + o 2 (g) →. Since c 2 h 5 oh contains carbon, co. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. (2) know that the combustion products. Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. Here is a brief overview of the solution steps before doing some examples:
The Reaction Of Combustion
How To Find The Product Of A Combustion Reaction Identify the reactants and products. Read through the given information in the problem for the chemical reaction. (1) identify the reaction as combustion: What is formed in any combustion reaction? Identify the reactants and products. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. (2) know that the combustion products. Combustion is a reaction in which a substance reacts with oxygen gas. Since c 2 h 5 oh contains carbon, co. In general chemistry, it is one of the main. How to figure out the right (or product side): What is needed for a combustion reaction to take place? Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. C 2 h 5 oh (l) + o 2 (g) →. 1) determine the grams of each element present in the original. Here is a brief overview of the solution steps before doing some examples:
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID5604352 How To Find The Product Of A Combustion Reaction Since c 2 h 5 oh contains carbon, co. (1) identify the reaction as combustion: Here is a brief overview of the solution steps before doing some examples: Read through the given information in the problem for the chemical reaction. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. (2). How To Find The Product Of A Combustion Reaction.
From ar.inspiredpencil.com
Combustion Reaction Examples How To Find The Product Of A Combustion Reaction (1) identify the reaction as combustion: How to figure out the right (or product side): A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. (2) know that the combustion products. What is formed in any combustion reaction? C 2 h 5 oh (l) + o 2 (g) →. Since c. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Combustion Analysis Sample YouTube How To Find The Product Of A Combustion Reaction Read through the given information in the problem for the chemical reaction. How to figure out the right (or product side): Here is a brief overview of the solution steps before doing some examples: What is needed for a combustion reaction to take place? What is formed in any combustion reaction? Since c 2 h 5 oh contains carbon, co.. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems YouTube How To Find The Product Of A Combustion Reaction What is formed in any combustion reaction? Here is a brief overview of the solution steps before doing some examples: Identify the reactants and products. (2) know that the combustion products. Combustion is a reaction in which a substance reacts with oxygen gas. How to figure out the right (or product side): C 2 h 5 oh (l) + o. How To Find The Product Of A Combustion Reaction.
From www.nagwa.com
Question Video Calculating the Enthalpy Change for the Reaction between Phenol and Diatomic How To Find The Product Of A Combustion Reaction How to figure out the right (or product side): C 2 h 5 oh (l) + o 2 (g) →. Here is a brief overview of the solution steps before doing some examples: (1) identify the reaction as combustion: Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. Use the masses and molar masses of the combustion products,. How To Find The Product Of A Combustion Reaction.
From www.slideserve.com
PPT Combustion PowerPoint Presentation, free download ID5525141 How To Find The Product Of A Combustion Reaction In general chemistry, it is one of the main. Since c 2 h 5 oh contains carbon, co. (2) know that the combustion products. Identify the reactants and products. Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. (1) identify the reaction as combustion: Here. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Identifying the reactants and products of an equation YouTube How To Find The Product Of A Combustion Reaction 1) determine the grams of each element present in the original. C 2 h 5 oh (l) + o 2 (g) →. (2) know that the combustion products. Since c 2 h 5 oh contains carbon, co. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. A combustion reaction is an exothermic chemical reaction between a fuel and. How To Find The Product Of A Combustion Reaction.
From organicvsa.weebly.com
Combustion equations 4 chemical equations and stoichiometry organicvsa How To Find The Product Of A Combustion Reaction Since c 2 h 5 oh contains carbon, co. How to figure out the right (or product side): Read through the given information in the problem for the chemical reaction. C 2 h 5 oh (l) + o 2 (g) →. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product.. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Empirical formula combustion YouTube How To Find The Product Of A Combustion Reaction What is formed in any combustion reaction? Since c 2 h 5 oh contains carbon, co. Identify the reactants and products. How to figure out the right (or product side): What is needed for a combustion reaction to take place? A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Read. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Empirical Formula from Combustion Analysis (Example) YouTube How To Find The Product Of A Combustion Reaction Since c 2 h 5 oh contains carbon, co. What is formed in any combustion reaction? 1) determine the grams of each element present in the original. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. Identify the reactants and products. C 2 h 5 oh (l) + o 2 (g) →. What is needed for a combustion. How To Find The Product Of A Combustion Reaction.
From www.showme.com
Combustion Reaction Science, Chemistry, Chemicalreactions ShowMe How To Find The Product Of A Combustion Reaction What is formed in any combustion reaction? C 2 h 5 oh (l) + o 2 (g) →. Since c 2 h 5 oh contains carbon, co. Identify the reactants and products. How to figure out the right (or product side): 1) determine the grams of each element present in the original. (1) identify the reaction as combustion: Here is. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
How to Find Product of Chemical Reaction Trick to Find product of Reaction YouTube How To Find The Product Of A Combustion Reaction Identify the reactants and products. Read through the given information in the problem for the chemical reaction. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. (2) know that the combustion products. C 2 h 5 oh (l) + o 2 (g) →. What is needed for a combustion reaction to take place? 1) determine the grams of. How To Find The Product Of A Combustion Reaction.
From worksheetzonecadies.z14.web.core.windows.net
Combustion Reaction Examples Chemistry How To Find The Product Of A Combustion Reaction Identify the reactants and products. What is formed in any combustion reaction? 1) determine the grams of each element present in the original. C 2 h 5 oh (l) + o 2 (g) →. (1) identify the reaction as combustion: What is needed for a combustion reaction to take place? Since c 2 h 5 oh contains carbon, co. Use. How To Find The Product Of A Combustion Reaction.
From www.learnaboutair.com
Combustion Learning about Air Quality How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. C 2 h 5 oh (l) + o 2 (g) →. (1) identify the reaction as combustion: Use the masses and molar masses of the combustion products, co 2 and. How To Find The Product Of A Combustion Reaction.
From study.com
Predicting the Products of a Combustion Reaction Chemistry How To Find The Product Of A Combustion Reaction Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. 1) determine the grams of each element present in the original. Here is a brief overview of the solution steps before doing some examples: Combustion is a reaction in which a substance reacts with oxygen gas.. How To Find The Product Of A Combustion Reaction.
From www.chegg.com
Solved = Predicting the products of a combustion reaction How To Find The Product Of A Combustion Reaction In general chemistry, it is one of the main. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Read through the given information in the problem for the chemical reaction. How to figure out the right (or product side): Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side.. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Balancing Combustion Reactions Chemistry Tutorial YouTube How To Find The Product Of A Combustion Reaction Read through the given information in the problem for the chemical reaction. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Combustion is a reaction in which a substance reacts with oxygen gas. (2) know that the combustion products. 1) determine the grams of each element present in the original.. How To Find The Product Of A Combustion Reaction.
From treatybottle13.pythonanywhere.com
Simple Combustion Reaction Octane Physics Formulas Of Class 12 How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Identify the reactants and products. Read through the given information in the problem for the chemical reaction. (1) identify the reaction as combustion: C 2 h 5 oh (l) + o 2 (g) →. Here is a brief overview of the. How To Find The Product Of A Combustion Reaction.
From www.thoughtco.com
What Is a Combustion Reaction? Definition and Examples How To Find The Product Of A Combustion Reaction What is formed in any combustion reaction? Read through the given information in the problem for the chemical reaction. Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. Since c 2 h 5 oh contains carbon, co. In general chemistry, it is one of the. How To Find The Product Of A Combustion Reaction.
From www.sciencephoto.com
Combustion reaction, illustration Stock Image F027/1851 Science Photo Library How To Find The Product Of A Combustion Reaction Since c 2 h 5 oh contains carbon, co. Here is a brief overview of the solution steps before doing some examples: 1) determine the grams of each element present in the original. What is needed for a combustion reaction to take place? Combustion is a reaction in which a substance reacts with oxygen gas. C 2 h 5 oh. How To Find The Product Of A Combustion Reaction.
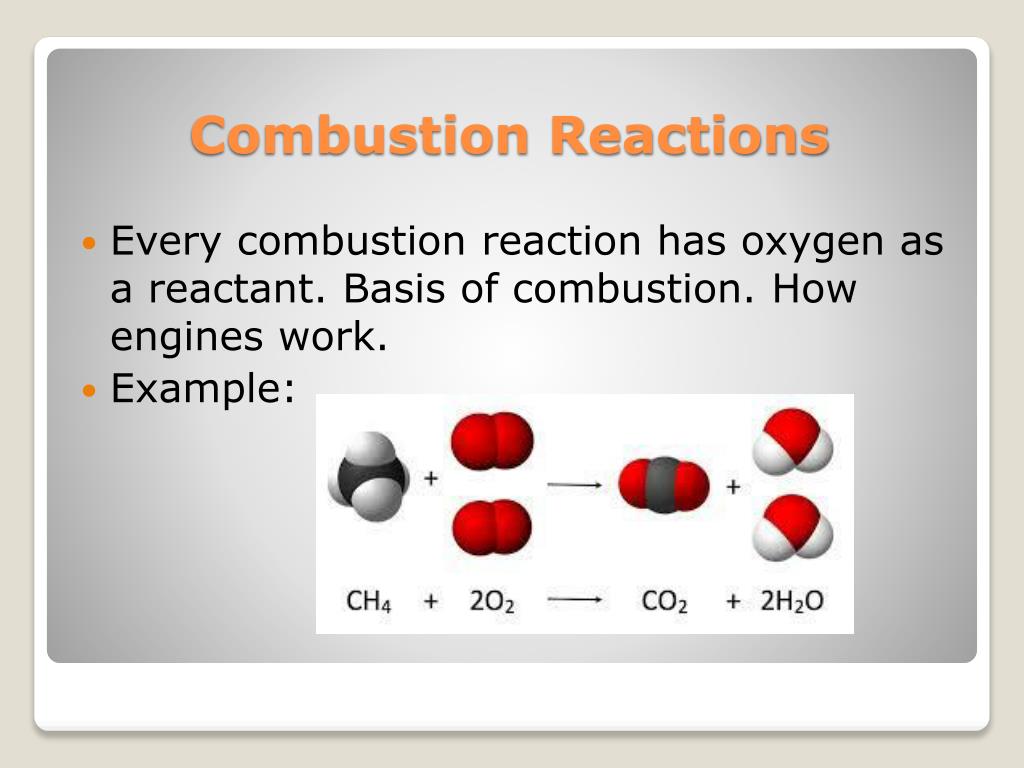
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID4816126 How To Find The Product Of A Combustion Reaction Combustion is a reaction in which a substance reacts with oxygen gas. C 2 h 5 oh (l) + o 2 (g) →. 1) determine the grams of each element present in the original. Since c 2 h 5 oh contains carbon, co. Here is a brief overview of the solution steps before doing some examples: Read through the given. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Percent Excess Air YouTube How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. What is formed in any combustion reaction? 1) determine the grams of each element present in the original. Since c 2 h 5 oh contains carbon, co. How to figure out the right (or product side): (1) identify the reaction as. How To Find The Product Of A Combustion Reaction.
From www.thoughtco.com
Main Kinds of Chemical Reactions How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Identify the reactants and products. How to figure out the right (or product side): 1) determine the grams of each element present in the original. What is needed for a combustion reaction to take place? Since c 2 h 5 oh. How To Find The Product Of A Combustion Reaction.
From gavynyouthbooth.blogspot.com
Describe What Happens in a Combustion Reaction How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Since c 2 h 5 oh contains carbon, co. (1) identify the reaction as combustion: Combustion is a reaction in which a substance reacts with oxygen gas. What is needed for a combustion reaction to take place? Read through the given. How To Find The Product Of A Combustion Reaction.
From studylib.net
20 Write a balanced equation for the complete combustion of each How To Find The Product Of A Combustion Reaction Identify the reactants and products. What is needed for a combustion reaction to take place? Since c 2 h 5 oh contains carbon, co. C 2 h 5 oh (l) + o 2 (g) →. 1) determine the grams of each element present in the original. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer. How To Find The Product Of A Combustion Reaction.
From worksheetlistfro.z13.web.core.windows.net
The Reaction Of Combustion How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. (2) know that the combustion products. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. In general chemistry, it is one of the main. C 2 h 5 oh (l) + o 2 (g) →. How to figure out. How To Find The Product Of A Combustion Reaction.
From sciencenotes.org
Combustion Reaction Definition and Examples How To Find The Product Of A Combustion Reaction Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. C 2 h 5 oh (l) + o 2 (g) →. Identify the reactants and products. How to figure out the right (or product side): Here is a brief overview. How To Find The Product Of A Combustion Reaction.
From preparatorychemistry.com
Combustion Analysis How To Find The Product Of A Combustion Reaction How to figure out the right (or product side): 1) determine the grams of each element present in the original. (2) know that the combustion products. Read through the given information in the problem for the chemical reaction. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Here is a. How To Find The Product Of A Combustion Reaction.
From www.pinterest.co.kr
Combustion Reaction Definition, Characteristics & Examples Carbon Dioxide, Redox Reactions How To Find The Product Of A Combustion Reaction Since c 2 h 5 oh contains carbon, co. C 2 h 5 oh (l) + o 2 (g) →. How to figure out the right (or product side): In general chemistry, it is one of the main. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. What is formed. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Combustion Reactions YouTube How To Find The Product Of A Combustion Reaction Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Since c 2 h 5 oh contains carbon, co. What is formed in any combustion reaction? Read. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Chemical Reactions (3 of 11) Combustion Reactions, An Explanation YouTube How To Find The Product Of A Combustion Reaction In general chemistry, it is one of the main. What is needed for a combustion reaction to take place? 1) determine the grams of each element present in the original. Identify the reactants and products. Determine if oxygen ( {eq}o_2 {/eq}) is on the reactants side. C 2 h 5 oh (l) + o 2 (g) →. (1) identify the. How To Find The Product Of A Combustion Reaction.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry How To Find The Product Of A Combustion Reaction A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. Since c 2 h 5 oh contains carbon, co. C 2 h 5 oh (l) + o 2 (g) →. How to figure out the right (or product side): Combustion is a reaction in which a substance reacts with oxygen gas.. How To Find The Product Of A Combustion Reaction.
From www.youtube.com
Advanced Combustion Reactions Honors Chemistry Ez Academy YouTube How To Find The Product Of A Combustion Reaction C 2 h 5 oh (l) + o 2 (g) →. Use the masses and molar masses of the combustion products, co 2 and h 2 o, to calculate the masses of carbon and hydrogen. Combustion is a reaction in which a substance reacts with oxygen gas. Since c 2 h 5 oh contains carbon, co. What is formed in. How To Find The Product Of A Combustion Reaction.
From www.slideserve.com
PPT COMBUSTION REACTIONS PowerPoint Presentation, free download ID4816126 How To Find The Product Of A Combustion Reaction In general chemistry, it is one of the main. C 2 h 5 oh (l) + o 2 (g) →. Identify the reactants and products. What is formed in any combustion reaction? Here is a brief overview of the solution steps before doing some examples: A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that. How To Find The Product Of A Combustion Reaction.
From www.shalom-education.com
Fuels and Combustion KS3 Chemistry Revision How To Find The Product Of A Combustion Reaction Identify the reactants and products. A combustion reaction is an exothermic chemical reaction between a fuel and an oxidizer that forms an oxidized product. What is needed for a combustion reaction to take place? (2) know that the combustion products. In general chemistry, it is one of the main. C 2 h 5 oh (l) + o 2 (g) →.. How To Find The Product Of A Combustion Reaction.