Latent Heat Lab . ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. the latent heat of fusion (associated with the melting of a solid) is defined by: energy is required to change water from a solid to a liquid, i.e. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. Lf = heat required to melt 1g of solid. we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to.
from www.studocu.com
In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. the latent heat of fusion (associated with the melting of a solid) is defined by: ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. energy is required to change water from a solid to a liquid, i.e. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. Lf = heat required to melt 1g of solid.
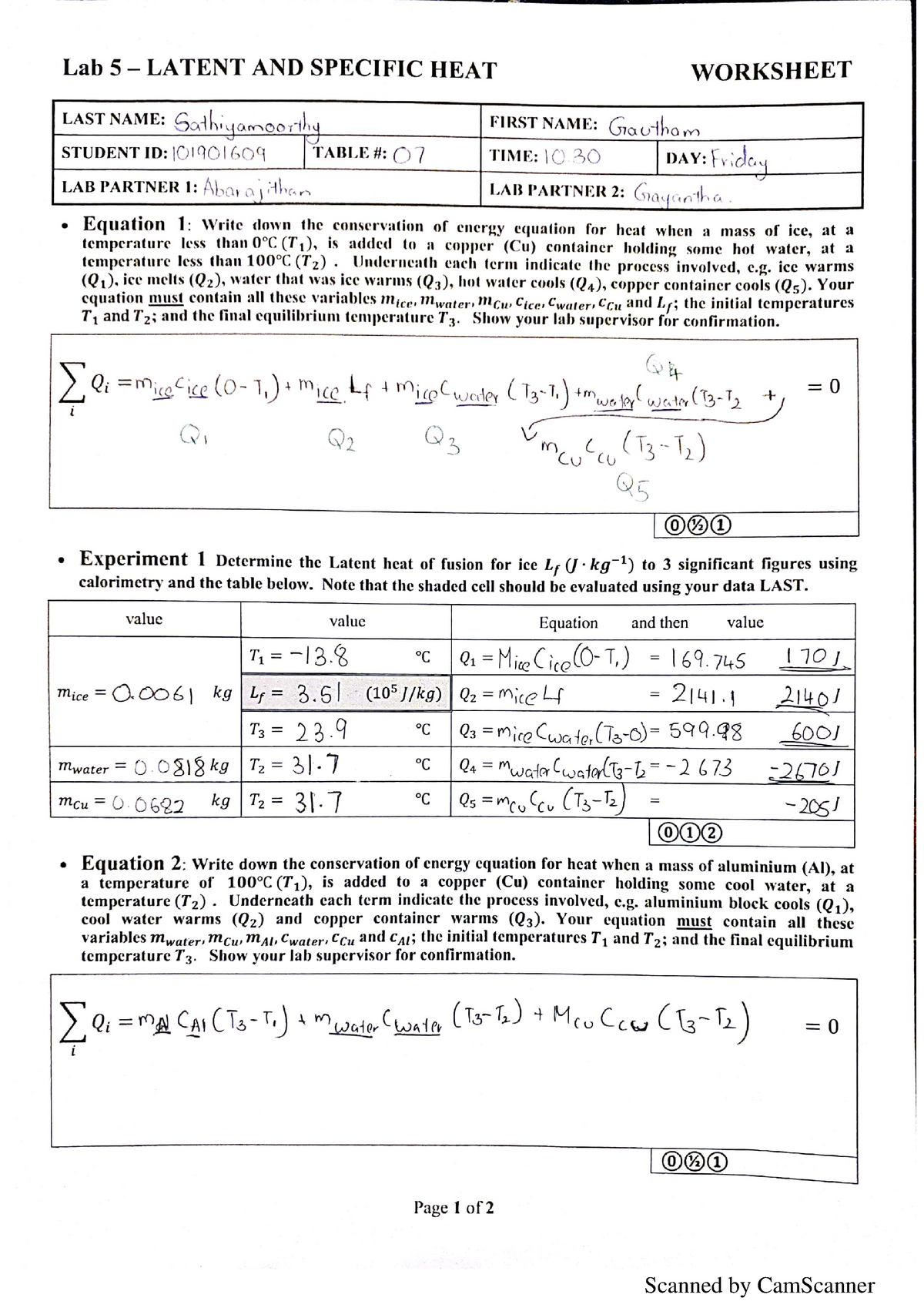
Lab 5 Latent & specific heat Lab 5 LATENT AND SPECIFIC HEAT
Latent Heat Lab In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; energy is required to change water from a solid to a liquid, i.e. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. the latent heat of fusion (associated with the melting of a solid) is defined by: the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. Lf = heat required to melt 1g of solid. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e.
From www.slideserve.com
PPT 146 Latent Heat PowerPoint Presentation, free download ID4043874 Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. energy is required to change water from a solid to a liquid, i.e. the latent heat of fusion (associated with the melting of a solid) is defined by: the latent heat of melting. Latent Heat Lab.
From studylib.net
Latent Heat of Fusion Latent Heat Lab the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. energy is required to change water from a solid to a liquid, i.e. we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass. Latent Heat Lab.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy Latent Heat Lab the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. the latent heat of fusion (associated with the melting of a solid) is defined by: in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and. Latent Heat Lab.
From studylib.net
LAB 05 LATENT HEAT OF FUSION Latent Heat Lab the latent heat of fusion (associated with the melting of a solid) is defined by: ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling. Latent Heat Lab.
From www.studypool.com
SOLUTION 202011250649168 Lab 8 Latent Heat And Specific Heat 1 Studypool Latent Heat Lab in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. energy is required to change water from a solid to a liquid, i.e. Lf = heat required to melt. Latent Heat Lab.
From www.slideshare.net
Lab 4 (specific latent heat) Latent Heat Lab the latent heat of fusion (associated with the melting of a solid) is defined by: explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; energy is required to change water from a solid to a liquid, i.e. the latent heat of melting l (j/kg), also known as the latent. Latent Heat Lab.
From www.scribd.com
Lab 8 Latent Heat vs. Sensible Heat PDF Latent Heat Heat Latent Heat Lab the latent heat of fusion (associated with the melting of a solid) is defined by: we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the. Latent Heat Lab.
From www.youtube.com
exp to find specific latent heat of fusion of ice YouTube Latent Heat Lab Lf = heat required to melt 1g of solid. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. energy is required to. Latent Heat Lab.
From www.studocu.com
Lab 11 Heat Capacity and Latent Heat The first part of this lab Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. the latent heat of melting l (j/kg), also known as the latent. Latent Heat Lab.
From www.chegg.com
Solved Latent Heat of Fusion Lab Instructions In this lab Latent Heat Lab in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. Lf = heat required to melt 1g of solid. In this experiment you will. Latent Heat Lab.
From www.scribd.com
Specific Heat Lab Report PDF Heat Latent Heat Latent Heat Lab energy is required to change water from a solid to a liquid, i.e. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. in this video, we'll. Latent Heat Lab.
From www.nachi.org
Latent Heat of Vaporization Inspection Gallery InterNACHI® Latent Heat Lab In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is. Latent Heat Lab.
From www.studocu.com
Lab 1 Specific Heat Capacity and Specific Latent heat Lab 1 Latent Heat Lab explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. we define the latent heat of fusion of ice lf as the amount of heat required to melt. Latent Heat Lab.
From www.studocu.com
Specific Latent Fusion of Heat Lab Specific Latent Fusion of Heat Lab Latent Heat Lab energy is required to change water from a solid to a liquid, i.e. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; Lf = heat required to melt 1g of solid. ๏ observe. Latent Heat Lab.
From science.clemson.edu
223 Physics Lab Specific and Latent Heat Latent Heat Lab Lf = heat required to melt 1g of solid. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. explain changes in. Latent Heat Lab.
From www.scribd.com
TITLE Latent Heat Lab Number 9 DATE February 22, 2018 AIM To Latent Heat Lab energy is required to change water from a solid to a liquid, i.e. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. In this experiment you will try. Latent Heat Lab.
From www.scribd.com
Latent Heat Lab Download Free PDF Latent Heat Heat Latent Heat Lab ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. energy is required to change water from a solid to a liquid,. Latent Heat Lab.
From www.slideserve.com
PPT Latent Heat PowerPoint Presentation, free download ID5759776 Latent Heat Lab In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. Lf = heat required to melt 1g of solid. explain changes in heat during changes of state, and describe. Latent Heat Lab.
From www.youtube.com
Physics 47 Latent Heat Lab YouTube Latent Heat Lab in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. explain changes in heat during changes of state, and describe latent heats of. Latent Heat Lab.
From www.chegg.com
Solved Latent Heat of Fusion Lab Instructions In this lab Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is. Latent Heat Lab.
From www.studocu.com
Lab 5 Latent & specific heat Lab 5 LATENT AND SPECIFIC HEAT Latent Heat Lab explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. Lf = heat required to melt 1g of solid. ๏ observe the transfer of heat energy from warm water. Latent Heat Lab.
From www.sutori.com
3. Adding steam to the water until it reached 38 degrees Celsius Latent Heat Lab in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. the latent heat of fusion (associated with the melting of a solid) is defined by: explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; In this experiment you. Latent Heat Lab.
From www.slideserve.com
PPT Temperature and Heat PowerPoint Presentation ID3080166 Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. Lf = heat required to melt 1g of solid. In this experiment you. Latent Heat Lab.
From alevelphysicsnotes.com
Mr Toogood Physics Latent heat Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating. Latent Heat Lab.
From www.nagwa.com
Lesson Specific Latent Heat Nagwa Latent Heat Lab in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. Lf = heat required to melt 1g of solid. explain changes in heat during changes of state, and describe. Latent Heat Lab.
From www.youtube.com
LATENT HEAT, SPECIFIC LATENT HEAT AND ITS UNIT YouTube Latent Heat Lab Lf = heat required to melt 1g of solid. energy is required to change water from a solid to a liquid, i.e. we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. In this experiment you will try to measure the latent heat of fusion. Latent Heat Lab.
From www.youtube.com
8 3 Latent Heat Lab Activity YouTube Latent Heat Lab energy is required to change water from a solid to a liquid, i.e. the latent heat of fusion (associated with the melting of a solid) is defined by: ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. Lf = heat required to melt 1g. Latent Heat Lab.
From studylib.net
To Measure the Specific Latent heat of Fusion of Ice Latent Heat Lab ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. the latent heat of fusion (associated with the melting of a solid). Latent Heat Lab.
From brainly.in
draw labelled diagram of the experimental set up to the latent heat of Latent Heat Lab explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as. Latent Heat Lab.
From www.teachoo.com
Latent Heat of Vaporization and Fusion Definition Teachoo Latent Heat Lab ๏ observe the transfer of heat energy from warm water to ice ๏ measure the temperature change as ice is melted ๏. energy is required to change water from a solid to a liquid, i.e. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed. Latent Heat Lab.
From studiousguy.com
9 Latent Heat Examples in Daily Life StudiousGuy Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. explain changes in heat during changes of state, and describe latent heats of fusion and vaporization; Lf = heat required to melt 1g of solid. the latent heat of fusion (associated with the melting. Latent Heat Lab.
From www.slideshare.net
Lab 4 (specific latent heat) PDF Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and cooling costs in. the latent heat of fusion (associated with the melting of a solid). Latent Heat Lab.
From www.docsity.com
Lab 12 Latent Heat of Fusion Study Guides, Projects, Research Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. energy is required to change water from a solid to a liquid, i.e. In this experiment you will try to measure the latent heat of fusion of ice (lhice), the. Lf = heat required to. Latent Heat Lab.
From www.studocu.com
106 Specific Heat E107 Latent Heat of Fusion Laboratory Report 106 Latent Heat Lab we define the latent heat of fusion of ice lf as the amount of heat required to melt a unit mass ice, i.e. the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. energy is required to change water from a solid to a. Latent Heat Lab.
From www.youtube.com
GCSE PHYSICS HEAT LESSON 22 latent heat fusion YouTube Latent Heat Lab the latent heat of fusion (associated with the melting of a solid) is defined by: the latent heat of melting l (j/kg), also known as the latent heat (or enthalpy) of fusion, is the heat energy needed to. in this video, we'll explain the concept of latent heat and see how it can dramatically reduce heating and. Latent Heat Lab.