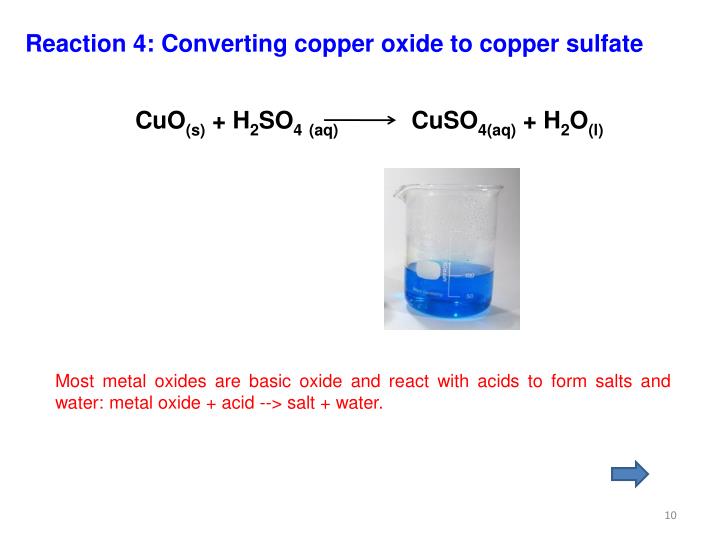
Copper (Ii) Oxide + Sulphuric Acid . Improved method with video support. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. — today we will be reacting copper (ii) oxide with sulfuric acid. Reacting copper (ii) oxide with sulfuric acid. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. In association with nuffield foundation. The copper takes the [o] and. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. — what is the balanced equation for sulphuric acid in copper oxide? ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. Preparing a soluble salt offers a safer method for this experiment using a water bath.
from www.slideserve.com
mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. In association with nuffield foundation. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. Improved method with video support. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. — today we will be reacting copper (ii) oxide with sulfuric acid. — what is the balanced equation for sulphuric acid in copper oxide? ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one.
PPT Laboratory 02 The Discovery of Chemical Change Through the
Copper (Ii) Oxide + Sulphuric Acid — today we will be reacting copper (ii) oxide with sulfuric acid. Improved method with video support. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. — today we will be reacting copper (ii) oxide with sulfuric acid. The copper takes the [o] and. Reacting copper (ii) oxide with sulfuric acid. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. — what is the balanced equation for sulphuric acid in copper oxide? mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. Preparing a soluble salt offers a safer method for this experiment using a water bath. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. In association with nuffield foundation.
From www.diomedia.com
STOCK IMAGE, , JF0695, 01B473E8 , Science Source Search Stock Photos Copper (Ii) Oxide + Sulphuric Acid ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. — today we will be reacting copper (ii) oxide with sulfuric acid. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Preparing. Copper (Ii) Oxide + Sulphuric Acid.
From questions-in.kunduz.com
The salt copper(II) sulfate can be prepar... Physical Chemistry Copper (Ii) Oxide + Sulphuric Acid The copper takes the [o] and. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. — today we will be reacting copper (ii) oxide with sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to. Copper (Ii) Oxide + Sulphuric Acid.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper (Ii) Oxide + Sulphuric Acid ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. —. Copper (Ii) Oxide + Sulphuric Acid.
From mungfali.com
Copper Oxide And Sulfuric Acid Copper (Ii) Oxide + Sulphuric Acid cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. Preparing a soluble salt offers a safer method for this experiment using a water bath. Improved method with video support. In this experiment an. Copper (Ii) Oxide + Sulphuric Acid.
From copperoxideandsulfuricacid.wordpress.com
copperoxideandsulfuricacid Just another site Copper (Ii) Oxide + Sulphuric Acid — today we will be reacting copper (ii) oxide with sulfuric acid. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. — what is the balanced equation for sulphuric acid in copper oxide? Reacting copper (ii). Copper (Ii) Oxide + Sulphuric Acid.
From dxojfqcbd.blob.core.windows.net
Copper Ii Oxide To Copper Ii Sulfate Equation at Gloria Schirmer blog Copper (Ii) Oxide + Sulphuric Acid — today we will be reacting copper (ii) oxide with sulfuric acid. In association with nuffield foundation. Improved method with video support. The copper takes the [o] and. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. In this experiment, students react an insoluble metal. Copper (Ii) Oxide + Sulphuric Acid.
From www.youtube.com
Make Copper II Sulfate YouTube Copper (Ii) Oxide + Sulphuric Acid Improved method with video support. — what is the balanced equation for sulphuric acid in copper oxide? — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. Reacting copper (ii) oxide. Copper (Ii) Oxide + Sulphuric Acid.
From www.youtube.com
neutralisation of sulphuric acid with copper oxide.mp4 YouTube Copper (Ii) Oxide + Sulphuric Acid In association with nuffield foundation. Improved method with video support. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. Preparing. Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
Copper sulphate formation. Test tube of copper (II) sulphate (blue) and Copper (Ii) Oxide + Sulphuric Acid cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. . Copper (Ii) Oxide + Sulphuric Acid.
From www.echemi.com
Why is it important for the amount of copper(II) oxide to be greater Copper (Ii) Oxide + Sulphuric Acid In association with nuffield foundation. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. The copper takes the [o] and. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. — what. Copper (Ii) Oxide + Sulphuric Acid.
From dillon-well-griffin.blogspot.com
What Does Copper Oxide and Sulfuric Acid Make Copper (Ii) Oxide + Sulphuric Acid — what is the balanced equation for sulphuric acid in copper oxide? In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. In association with nuffield foundation. ores containing cupric oxide (cuo) are commonly reacted. Copper (Ii) Oxide + Sulphuric Acid.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper (Ii) Oxide + Sulphuric Acid Preparing a soluble salt offers a safer method for this experiment using a water bath. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. In this experiment, students react an insoluble. Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
black copper oxide dissolved in sulphuric acid produces copper Stock Copper (Ii) Oxide + Sulphuric Acid Reacting copper (ii) oxide with sulfuric acid. Preparing a soluble salt offers a safer method for this experiment using a water bath. The copper takes the [o] and. Improved method with video support. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. In this experiment an insoluble metal oxide is reacted. Copper (Ii) Oxide + Sulphuric Acid.
From www.youtube.com
Mixing copper oxide with distilled water and sulfuric acid MVI 6706 Copper (Ii) Oxide + Sulphuric Acid — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Improved method with video support. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis). Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
copper oxide and sulphuric acid form copper sulphate solution which Copper (Ii) Oxide + Sulphuric Acid — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. — today we will be reacting copper (ii) oxide with sulfuric acid. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. Reacting copper (ii) oxide with sulfuric acid. Improved method with video support. In association. Copper (Ii) Oxide + Sulphuric Acid.
From daisy-yersblogchoi.blogspot.com
Word Equation for Copper Oxide and Sulfuric Acid Copper (Ii) Oxide + Sulphuric Acid In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. cuo + h2so4 = cuo4s + h2o is a double. Copper (Ii) Oxide + Sulphuric Acid.
From www.sciencephoto.com
Copper (II) oxide and sulphuric acid Stock Image C037/2520 Copper (Ii) Oxide + Sulphuric Acid — what is the balanced equation for sulphuric acid in copper oxide? mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Improved method with video support. Preparing a soluble salt offers a safer method for this experiment using a water bath.. Copper (Ii) Oxide + Sulphuric Acid.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper (Ii) Oxide + Sulphuric Acid In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. — today we will be reacting copper (ii) oxide with sulfuric acid. The copper takes the [o] and. mixing copper oxide and sulphuric acid is an experiment. Copper (Ii) Oxide + Sulphuric Acid.
From www.sciencephoto.com
Copper sulphate formation Stock Image C011/4521 Science Photo Library Copper (Ii) Oxide + Sulphuric Acid The copper takes the [o] and. Reacting copper (ii) oxide with sulfuric acid. — what is the balanced equation for sulphuric acid in copper oxide? — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. In. Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
black copper oxide dissolved in sulphuric acid produces copper sulphate Copper (Ii) Oxide + Sulphuric Acid Preparing a soluble salt offers a safer method for this experiment using a water bath. In association with nuffield foundation. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. Improved method with video support. — today we will be reacting copper (ii) oxide with sulfuric acid. cuo + h2so4. Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
Two copper oxide reactions. Heating copper oxide with dilute sulphuric Copper (Ii) Oxide + Sulphuric Acid — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Improved method with video support. — today we will be reacting copper (ii) oxide with sulfuric acid. — what is the balanced equation for sulphuric acid in copper oxide? Reacting copper (ii) oxide with sulfuric acid. mixing copper oxide and sulphuric acid. Copper (Ii) Oxide + Sulphuric Acid.
From kristophernpetton.blogspot.com
Copper Oxide and Sulfuric Acid Formula KristophernPetton Copper (Ii) Oxide + Sulphuric Acid — what is the balanced equation for sulphuric acid in copper oxide? Improved method with video support. Reacting copper (ii) oxide with sulfuric acid. — today we will be reacting copper (ii) oxide with sulfuric acid. The copper takes the [o] and. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which. Copper (Ii) Oxide + Sulphuric Acid.
From www.numerade.com
SOLVED I am required to find the molecular equation, ionic equation Copper (Ii) Oxide + Sulphuric Acid Improved method with video support. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. Reacting copper (ii) oxide with sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. — you can imagine concentrated sulfuric acid. Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
Copper oxide and sulphuric acid. Container of copper (II) oxide (left Copper (Ii) Oxide + Sulphuric Acid Reacting copper (ii) oxide with sulfuric acid. — what is the balanced equation for sulphuric acid in copper oxide? cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble. Copper (Ii) Oxide + Sulphuric Acid.
From www.sciencephoto.com
Copper (II) oxide reacts with sulfuric acid Stock Image C036/3130 Copper (Ii) Oxide + Sulphuric Acid Reacting copper (ii) oxide with sulfuric acid. The copper takes the [o] and. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. In association with nuffield foundation. — what is the balanced equation for sulphuric acid in copper oxide? In. Copper (Ii) Oxide + Sulphuric Acid.
From www.alamy.com
Beaker being used to add sulphuric acid to copper (II) oxide (black) at Copper (Ii) Oxide + Sulphuric Acid Reacting copper (ii) oxide with sulfuric acid. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. — what is the balanced equation for sulphuric acid in copper oxide? — you can imagine concentrated sulfuric acid as. Copper (Ii) Oxide + Sulphuric Acid.
From www.researchgate.net
(PDF) Sulphuric Acid BakeLeach Process for the Treatment of Mixed Copper (Ii) Oxide + Sulphuric Acid The copper takes the [o] and. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. Preparing a soluble salt offers a safer method for this experiment using a water bath. Improved method with. Copper (Ii) Oxide + Sulphuric Acid.
From nail.ftempo.com
Iron Nail In Copper Ii Sulfate Nail Ftempo Copper (Ii) Oxide + Sulphuric Acid ores containing cupric oxide (cuo) are commonly reacted with sulfuric acid to. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. — what is the balanced equation for sulphuric acid in copper oxide? mixing copper oxide and sulphuric acid is an experiment involving. Copper (Ii) Oxide + Sulphuric Acid.
From pixels.com
Copper (ii) Oxide Photograph by Martyn F. Chillmaid/science Photo Copper (Ii) Oxide + Sulphuric Acid — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. — today we will be reacting copper (ii) oxide with sulfuric acid. Reacting copper (ii) oxide with sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a. Copper (Ii) Oxide + Sulphuric Acid.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper (Ii) Oxide + Sulphuric Acid In association with nuffield foundation. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Improved method with video support. In this experiment, students react an insoluble metal oxide with a dilute acid to form a soluble salt. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is. Copper (Ii) Oxide + Sulphuric Acid.
From www.youtube.com
Reaction of copper oxide and sulfuric acid YouTube Copper (Ii) Oxide + Sulphuric Acid Improved method with video support. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. Reacting copper (ii) oxide with sulfuric acid. Preparing a soluble salt offers a safer method for this experiment using a water bath. — what is the balanced equation for sulphuric acid in copper oxide? mixing copper oxide and. Copper (Ii) Oxide + Sulphuric Acid.
From www.numerade.com
SOLVED When sulfuric acid and copper (II) oxide are allowed to react Copper (Ii) Oxide + Sulphuric Acid — today we will be reacting copper (ii) oxide with sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. ores containing cupric oxide (cuo) are commonly reacted. Copper (Ii) Oxide + Sulphuric Acid.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper (Ii) Oxide + Sulphuric Acid cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. — today we will be reacting copper (ii) oxide with sulfuric acid. — what is the balanced equation for sulphuric acid in copper oxide? The copper takes the [o] and. Improved method with video support.. Copper (Ii) Oxide + Sulphuric Acid.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052 Copper (Ii) Oxide + Sulphuric Acid mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. — what is the balanced equation for sulphuric acid in copper oxide? — today we will be reacting copper (ii) oxide with sulfuric acid. — you can imagine concentrated sulfuric. Copper (Ii) Oxide + Sulphuric Acid.
From www.numerade.com
Copper(II) sulfide is oxidized by molecular oxygen to produce gaseous Copper (Ii) Oxide + Sulphuric Acid — you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one. In this experiment an insoluble metal oxide is reacted with a dilute acid to form a soluble salt. mixing copper oxide. Copper (Ii) Oxide + Sulphuric Acid.