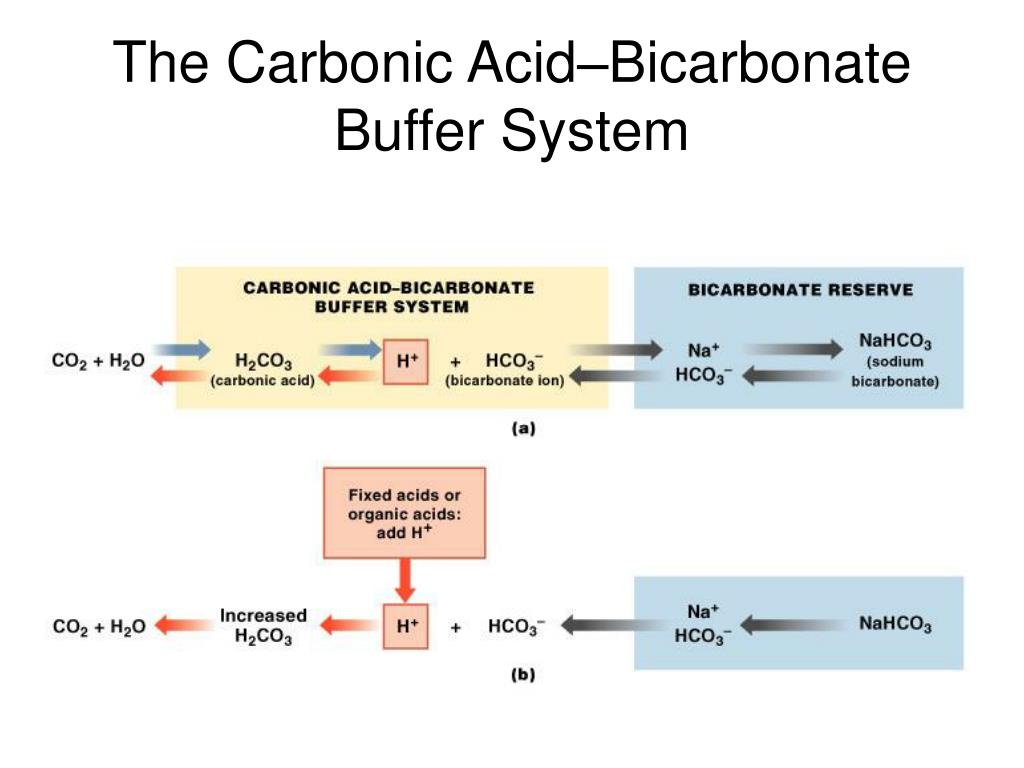
What Does The Bicarbonate Buffer System Do . Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. Firstly, it is the most plentiful buffer within the body;. the bicarbonate buffer system. — the bicarbonate system is important for two reasons. — key points. a buffer is a solution that resists sudden changes in ph. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The ph of the blood is maintained between 7.35 and 7.45 by an. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout.
from www.slideserve.com
Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the bicarbonate buffer system. Firstly, it is the most plentiful buffer within the body;. The ph of the blood is maintained between 7.35 and 7.45 by an. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. a buffer is a solution that resists sudden changes in ph. — key points. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — the bicarbonate system is important for two reasons.
PPT Chapter 26 Balance PowerPoint Presentation, free download ID
What Does The Bicarbonate Buffer System Do — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. a buffer is a solution that resists sudden changes in ph. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Firstly, it is the most plentiful buffer within the body;. — the bicarbonate system is important for two reasons. — key points. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph of the blood is maintained between 7.35 and 7.45 by an. the bicarbonate buffer system.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download What Does The Bicarbonate Buffer System Do — key points. Firstly, it is the most plentiful buffer within the body;. a buffer is a solution that resists sudden changes in ph. — the bicarbonate system is important for two reasons. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — the carbon dioxide formed during. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Chapter 24 Lecture Outline See PowerPoint Image Slides for all What Does The Bicarbonate Buffer System Do The ph of the blood is maintained between 7.35 and 7.45 by an. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Firstly, it is the most plentiful buffer within the body;. a buffer is. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2240409 What Does The Bicarbonate Buffer System Do — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. The ph of the blood is maintained between 7.35 and 7.45 by an. — the carbon dioxide formed during cellular respiration combines with water to create. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT pH and Biological Systems PowerPoint Presentation, free download What Does The Bicarbonate Buffer System Do — key points. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. Firstly, it is the most plentiful buffer. What Does The Bicarbonate Buffer System Do.
From study.com
Bicarbonate Buffer System Overview, Equation & Uses Video & Lesson What Does The Bicarbonate Buffer System Do — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. a buffer is a solution that resists sudden changes in ph. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — key points. Blood ph is regulated by multiple homeostatic mechanisms,. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Does The Bicarbonate Buffer System Do The ph of the blood is maintained between 7.35 and 7.45 by an. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — intracellular and extracellular buffers are the most immediate mechanism of defense against. What Does The Bicarbonate Buffer System Do.
From www.pinterest.ca
(MCAT favorite) VHY Bicarbonate Buffer System Medical student What Does The Bicarbonate Buffer System Do — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. a buffer is a solution that resists sudden changes in ph. — the carbon dioxide formed during cellular respiration combines with water. What Does The Bicarbonate Buffer System Do.
From quizlet.com
The bicarbonate buffer system Diagram Quizlet What Does The Bicarbonate Buffer System Do Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — the bicarbonate system is important for two reasons. The ph of the blood is maintained between 7.35 and 7.45 by an. — key points. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. — the. What Does The Bicarbonate Buffer System Do.
From www.reagent.co.uk
How Do Biological Buffers Work? The Science Blog What Does The Bicarbonate Buffer System Do — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. a buffer is a solution that resists sudden changes in ph. — the carbon dioxide formed during cellular respiration combines with water. What Does The Bicarbonate Buffer System Do.
From www.youtube.com
Carbonate Buffering YouTube What Does The Bicarbonate Buffer System Do — the bicarbonate system is important for two reasons. Firstly, it is the most plentiful buffer within the body;. — key points. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. the bicarbonate buffer system. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic.. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 What Does The Bicarbonate Buffer System Do Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. Firstly, it is the most plentiful buffer within the body;. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. a buffer. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Lecture Goals PowerPoint Presentation, free download ID3309237 What Does The Bicarbonate Buffer System Do the bicarbonate buffer system. Firstly, it is the most plentiful buffer within the body;. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. a buffer is a solution that resists sudden changes in ph. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. —. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Chapter 27, part 2 PowerPoint Presentation, free download ID What Does The Bicarbonate Buffer System Do Firstly, it is the most plentiful buffer within the body;. The ph of the blood is maintained between 7.35 and 7.45 by an. — key points. a buffer is a solution that resists sudden changes in ph. the bicarbonate buffer system. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. —. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Chapter 27 PowerPoint Presentation, free download ID3705071 What Does The Bicarbonate Buffer System Do — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. the bicarbonate buffer system. The ph of the blood is maintained between 7.35 and 7.45 by an. a buffer is a solution that resists sudden. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Does The Bicarbonate Buffer System Do Firstly, it is the most plentiful buffer within the body;. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Homeostasis PowerPoint Presentation, free download ID1562690 What Does The Bicarbonate Buffer System Do — the bicarbonate system is important for two reasons. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. a buffer is a solution that resists sudden changes in ph. — the bicarbonate buffer is the. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Figure 10.9 The respiratory cycle. PowerPoint Presentation ID What Does The Bicarbonate Buffer System Do — the bicarbonate system is important for two reasons. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Firstly, it is the most plentiful buffer within the body;. a buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35. What Does The Bicarbonate Buffer System Do.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and What Does The Bicarbonate Buffer System Do The ph of the blood is maintained between 7.35 and 7.45 by an. a buffer is a solution that resists sudden changes in ph. — key points. the bicarbonate buffer system. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — the carbon dioxide formed during cellular respiration. What Does The Bicarbonate Buffer System Do.
From www.youtube.com
Bicarbonate The Primary Buffer YouTube What Does The Bicarbonate Buffer System Do — the bicarbonate system is important for two reasons. — key points. a buffer is a solution that resists sudden changes in ph. the bicarbonate buffer system. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes. What Does The Bicarbonate Buffer System Do.
From quizdemurrages.z21.web.core.windows.net
How Bicarbonate Buffer System Works What Does The Bicarbonate Buffer System Do The ph of the blood is maintained between 7.35 and 7.45 by an. — key points. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. a buffer is a solution that resists sudden changes in ph. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the.. What Does The Bicarbonate Buffer System Do.
From www.chegg.com
Solved The Bicarbonate Buffering System Increase H What Does The Bicarbonate Buffer System Do — key points. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Firstly, it is the most plentiful buffer within the body;. a buffer is a solution that resists sudden changes in ph. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues. What Does The Bicarbonate Buffer System Do.
From slideplayer.com
The Urinary System. ppt download What Does The Bicarbonate Buffer System Do — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Firstly, it is the most plentiful buffer within the body;. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph of the blood is maintained between 7.35 and 7.45 by an. . What Does The Bicarbonate Buffer System Do.
From fieselina.blogspot.com
Carbonate Buffer System Explained carbonate What Does The Bicarbonate Buffer System Do — the bicarbonate system is important for two reasons. the bicarbonate buffer system. Firstly, it is the most plentiful buffer within the body;. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — the bicarbonate buffer. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Chapter 27 Fluid, Electrolyte and Acidbase Balance PowerPoint What Does The Bicarbonate Buffer System Do a buffer is a solution that resists sudden changes in ph. The ph of the blood is maintained between 7.35 and 7.45 by an. Firstly, it is the most plentiful buffer within the body;. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. — the bicarbonate system is important for two. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Carbonic AcidBicarbonate Buffering System PowerPoint What Does The Bicarbonate Buffer System Do — key points. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the bicarbonate buffer system. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Firstly, it is the most plentiful buffer within the body;. The ph of the blood. What Does The Bicarbonate Buffer System Do.
From www.youtube.com
Bicarbonate Buffer System YouTube What Does The Bicarbonate Buffer System Do — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. a buffer is a solution that resists sudden changes in ph. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in. What Does The Bicarbonate Buffer System Do.
From quizparaguayan.z4.web.core.windows.net
How Bicarbonate Buffer System Works What Does The Bicarbonate Buffer System Do Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the bicarbonate buffer system. — key points. a buffer is a solution that resists sudden changes in ph. Firstly, it is the most plentiful buffer within. What Does The Bicarbonate Buffer System Do.
From quizlet.com
Bicarbonate Buffer system Diagram Quizlet What Does The Bicarbonate Buffer System Do The ph of the blood is maintained between 7.35 and 7.45 by an. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. — key points. — the bicarbonate system is important for two reasons. Firstly, it is the most plentiful buffer within the body;. a buffer is a solution that. What Does The Bicarbonate Buffer System Do.
From www.pinterest.ca
bicarbonate buffer system, example of multiple equilibria Teaching What Does The Bicarbonate Buffer System Do a buffer is a solution that resists sudden changes in ph. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. — key points. The ph of the blood is maintained between 7.35 and 7.45 by an. Firstly,. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download What Does The Bicarbonate Buffer System Do — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. The ph of the blood is maintained between 7.35 and 7.45 by an. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. — the bicarbonate buffer is the primary buffering system of the if surrounding. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download What Does The Bicarbonate Buffer System Do — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. the bicarbonate buffer system. — the bicarbonate system is important for two reasons. — the bicarbonate buffer is the primary buffering system of the if surrounding the. What Does The Bicarbonate Buffer System Do.
From www.youtube.com
The bicarbonate buffer system YouTube What Does The Bicarbonate Buffer System Do the bicarbonate buffer system. — key points. — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. The ph of the blood is maintained between 7.35 and 7.45 by an. Firstly, it is the most plentiful buffer within the body;. — intracellular and extracellular buffers are the most immediate mechanism of. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Does The Bicarbonate Buffer System Do — the carbon dioxide formed during cellular respiration combines with water to create carbonic acid. Firstly, it is the most plentiful buffer within the body;. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Bicarbonate buffer system PowerPoint Presentation, free download What Does The Bicarbonate Buffer System Do — the bicarbonate system is important for two reasons. the bicarbonate buffer system. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. Firstly, it is the most plentiful buffer within the body;. — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. — key points.. What Does The Bicarbonate Buffer System Do.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation, free download ID2563527 What Does The Bicarbonate Buffer System Do — intracellular and extracellular buffers are the most immediate mechanism of defense against changes in systemic. the bicarbonate buffer system. — key points. — the bicarbonate buffer is the primary buffering system of the if surrounding the cells in tissues throughout. Blood ph is regulated by multiple homeostatic mechanisms, including chemical buffers, respiration, and the. The. What Does The Bicarbonate Buffer System Do.