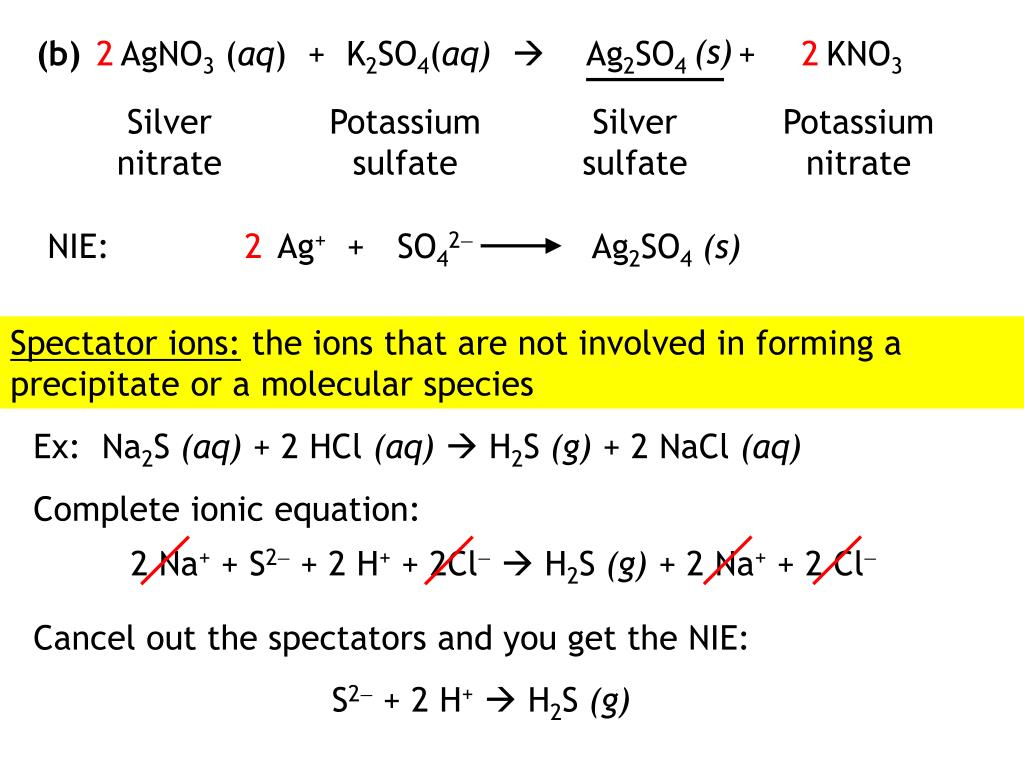
Zinc Nitrate + Potassium Iodide . Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): if you're seeing this message, it means we're having trouble loading external resources on our website. Molecular, complete ionic, and net ionic equations. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. If you're behind a web filter,. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. Reactions of lead (ii) nitrate and. Ap®︎/college chemistry > unit 4.
from dxomzyoyw.blob.core.windows.net
to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter,. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. Reactions of lead (ii) nitrate and. Ap®︎/college chemistry > unit 4.
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog
Zinc Nitrate + Potassium Iodide a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Zn (no3)2 + ki = zni2 + kno3 is a double displacement. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Molecular, complete ionic, and net ionic equations. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. if you're seeing this message, it means we're having trouble loading external resources on our website. Reactions of lead (ii) nitrate and. If you're behind a web filter,. Ap®︎/college chemistry > unit 4.
From www.numerade.com
SOLVED A solution of zinc nitrate is mixed with a solution of Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. If you're behind a web filter,. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Molecular, complete ionic, and net ionic equations. Reactions of lead (ii) nitrate and. zinc nitrate + potassium iodide. Zinc Nitrate + Potassium Iodide.
From www.indiamart.com
Zinc Nitrate Solution at Rs 165/litre Zinc Nitrate in Vadodara ID Zinc Nitrate + Potassium Iodide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. zinc nitrate + potassium iodide = zinc iodide. Zinc Nitrate + Potassium Iodide.
From www.indiamart.com
Zinc Nitrate at Rs 3750/unit Ram Darbar ID 22998737530 Zinc Nitrate + Potassium Iodide Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. If you're behind a web filter,. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Reactions of. Zinc Nitrate + Potassium Iodide.
From www.toppr.com
\"Reaction of potassium iodide solution with lead nitrate solution Zinc Nitrate + Potassium Iodide Zn (no3)2 + ki = zni2 + kno3 is a double displacement. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. if you're seeing this message, it means we're having trouble loading external resources on our website. to balance a chemical equation, enter an equation of a chemical reaction. Zinc Nitrate + Potassium Iodide.
From www.nanochemazone.ca
Zinc Nitrate Hexahydrate Nanochemazone CAS 10196186 Zinc Nitrate + Potassium Iodide Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Reactions of lead (ii) nitrate and. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. a. Zinc Nitrate + Potassium Iodide.
From ar.inspiredpencil.com
Potassium Iodide In Water Zinc Nitrate + Potassium Iodide zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Ap®︎/college chemistry > unit 4. Reactions of lead (ii) nitrate and. if you're seeing this message, it means we're having trouble loading external resources on our website. Zn (no3)2 + ki = zni2 + kno3 is a double. Zinc Nitrate + Potassium Iodide.
From stock.adobe.com
Zinc iodide or Zn2 iodide, powder. Chemical compound of zinc and iodine Zinc Nitrate + Potassium Iodide Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Reactions of lead (ii) nitrate and. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. If you're behind a web filter,. Ap®︎/college chemistry > unit 4. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. zinc nitrate +. Zinc Nitrate + Potassium Iodide.
From www.sciencecompany.com
Lab Grade Zinc Nitrate, 500g for sale. Buy from The Science Company. Zinc Nitrate + Potassium Iodide Reactions of lead (ii) nitrate and. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. if you're seeing this message, it means we're having trouble loading external resources on our website. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): zinc nitrate + potassium iodide = zinc iodide + potassium. Zinc Nitrate + Potassium Iodide.
From www.dreamstime.com
Zinc Iodide or Zn2 Iodide, White Powder. Chemical Compound of Zinc and Zinc Nitrate + Potassium Iodide If you're behind a web filter,. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Molecular, complete ionic, and net ionic equations. if you're seeing this message, it means we're having trouble loading external resources on our website. to balance a chemical equation, enter an equation of a chemical reaction and press the balance. Zinc Nitrate + Potassium Iodide.
From www.slideshare.net
Precipitates Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Reactions of lead (ii) nitrate and. If you're behind a web filter,.. Zinc Nitrate + Potassium Iodide.
From martlabpro.com
Lead 2 Nitrate And Potassium Iodide A Comprehensive Guide MartLabPro Zinc Nitrate + Potassium Iodide Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Molecular, complete ionic, and net ionic equations. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Reactions of lead (ii) nitrate and. write a balanced equation for the decomposition. Zinc Nitrate + Potassium Iodide.
From www.fishersci.com
MercuricPotassium Iodide TS, Mayer's Reagent, Ricca Chemical Fisher Zinc Nitrate + Potassium Iodide Reactions of lead (ii) nitrate and. If you're behind a web filter,. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Molecular, complete ionic, and net ionic equations. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): . Zinc Nitrate + Potassium Iodide.
From proper-cooking.info
Potassium Iodide Solution Formula Zinc Nitrate + Potassium Iodide — lead iodide precipitates when potassium iodide is mixed with lead nitrate. if you're seeing this message, it means we're having trouble loading external resources on our website. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. Ap®︎/college. Zinc Nitrate + Potassium Iodide.
From stock.adobe.com
Zinc iodid or iodide Zn2+ chemical compound of zinc and iodine on Zinc Nitrate + Potassium Iodide write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. to balance a chemical equation, enter an equation of a chemical reaction. Zinc Nitrate + Potassium Iodide.
From www.youtube.com
Potassium Iodide and Lead Nitrate Yellow PPT Double Displacement Zinc Nitrate + Potassium Iodide Reactions of lead (ii) nitrate and. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Ap®︎/college chemistry > unit 4. write. Zinc Nitrate + Potassium Iodide.
From www.teachoo.com
Double Displacement Reaction Definition, Examples, Types Teachoo Zinc Nitrate + Potassium Iodide a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): to balance a chemical equation, enter an equation of a chemical reaction and press the. Zinc Nitrate + Potassium Iodide.
From www.toppr.com
Write the balanced chemical equation the following reactions & identify Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): Zn (no3)2 + ki = zni2 + kno3 is a double displacement. If you're behind a web filter,. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. a precipitation. Zinc Nitrate + Potassium Iodide.
From www.ranksbooster.in
Identify the type of reactions taking place in each of the following Zinc Nitrate + Potassium Iodide If you're behind a web filter,. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. if you're seeing this message, it means we're having trouble loading external resources on our website. to balance a chemical equation, enter an equation of a chemical. Zinc Nitrate + Potassium Iodide.
From www.slideserve.com
PPT Predicting solubility PowerPoint Presentation, free download ID Zinc Nitrate + Potassium Iodide Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Ap®︎/college chemistry > unit 4. if you're seeing this message, it means we're having trouble loading external resources on our website. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two. Zinc Nitrate + Potassium Iodide.
From www.grainger.com
10139476, F.W. 319.22, Zinc Iodide, Purified 39H157Z1073500GM10 Zinc Nitrate + Potassium Iodide Zn (no3)2 + ki = zni2 + kno3 is a double displacement. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and.. Zinc Nitrate + Potassium Iodide.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Zinc Nitrate + Potassium Iodide If you're behind a web filter,. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Reactions of lead (ii) nitrate and. Ap®︎/college chemistry > unit 4. to balance a chemical equation, enter an. Zinc Nitrate + Potassium Iodide.
From exobevaom.blob.core.windows.net
Products Of Magnesium And Zinc Nitrate at Roberto Olson blog Zinc Nitrate + Potassium Iodide Reactions of lead (ii) nitrate and. Ap®︎/college chemistry > unit 4. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. to balance a chemical equation, enter an equation of. Zinc Nitrate + Potassium Iodide.
From www.numerade.com
SOLVED Write the chemical formula of magnesium oxide, lead nitrate Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. to balance a chemical equation, enter an equation of a chemical. Zinc Nitrate + Potassium Iodide.
From www.alamy.com
ZnI2 Zinc iodide. Chemical compound. CAS number 10139476 Stock Zinc Nitrate + Potassium Iodide a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. Molecular, complete ionic, and net ionic equations. Ap®︎/college chemistry > unit 4. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): if you're seeing this message, it means we're having. Zinc Nitrate + Potassium Iodide.
From chemistryexperimentphotogallery.blogspot.com
Chemistry Experiment Photo Gallery chapter 8 Form 4 Salts Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Molecular, complete ionic, and net ionic equations. If you're behind a web filter,. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. — lead iodide precipitates when potassium iodide is mixed with lead. Zinc Nitrate + Potassium Iodide.
From dxovcakol.blob.core.windows.net
If You Dissolve Lead Ii Nitrate And Potassium Iodide at Carmen Acker blog Zinc Nitrate + Potassium Iodide zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. If you're behind a web filter,. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. if you're seeing this message, it means we're having trouble. Zinc Nitrate + Potassium Iodide.
From first-labs.com
Zinc nitrate hexahydrate Zinc Nitrate + Potassium Iodide Molecular, complete ionic, and net ionic equations. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. Reactions of lead (ii) nitrate and. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Reactions of copper (ii) sulfate. Zinc Nitrate + Potassium Iodide.
From hygrotech.co.za
ZINC NITRATE Hygrotech Zinc Nitrate + Potassium Iodide zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Molecular, complete ionic, and net ionic equations. if. Zinc Nitrate + Potassium Iodide.
From www.numerade.com
SOLVEDA solution of zinc nitrate is mixed with a solution of potassium Zinc Nitrate + Potassium Iodide Molecular, complete ionic, and net ionic equations. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Reactions of lead (ii) nitrate and. Ap®︎/college chemistry > unit 4. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate.. Zinc Nitrate + Potassium Iodide.
From www.dreamstime.com
Zinc Iodide or Zn2 Iodide, White Powder. Chemical Compound of Zinc and Zinc Nitrate + Potassium Iodide zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. If you're behind a. Zinc Nitrate + Potassium Iodide.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead iodide Fundamental Zinc Nitrate + Potassium Iodide Reactions of lead (ii) nitrate and. If you're behind a web filter,. Ap®︎/college chemistry > unit 4. Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. a precipitation reaction. Zinc Nitrate + Potassium Iodide.
From www.pw.live
Zinc Iodide Formula, Structure, Properties And Uses Zinc Nitrate + Potassium Iodide Reactions of copper (ii) sulfate solution and zinc \(\pageindex{f}\): If you're behind a web filter,. Molecular, complete ionic, and net ionic equations. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. if you're seeing this message, it means we're having trouble loading external resources on our website. Ap®︎/college chemistry > unit 4. write a. Zinc Nitrate + Potassium Iodide.
From chemcraft.su
Zinc nitrate hexahydrate, 98 pure chemcraft.su Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. zinc nitrate + potassium iodide = zinc iodide + potassium nitrate. — lead iodide precipitates when potassium iodide is mixed with lead nitrate. write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen, molecular oxygen, and. if you're seeing this message, it means we're having trouble loading. Zinc Nitrate + Potassium Iodide.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube Zinc Nitrate + Potassium Iodide If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. Reactions of lead (ii) nitrate and. a precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Ap®︎/college chemistry. Zinc Nitrate + Potassium Iodide.
From www.youtube.com
Lead Nitrate + Potassium Iodide YouTube Zinc Nitrate + Potassium Iodide Ap®︎/college chemistry > unit 4. if you're seeing this message, it means we're having trouble loading external resources on our website. Reactions of lead (ii) nitrate and. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Zn (no3)2 + ki = zni2 + kno3 is a double displacement. Reactions of. Zinc Nitrate + Potassium Iodide.