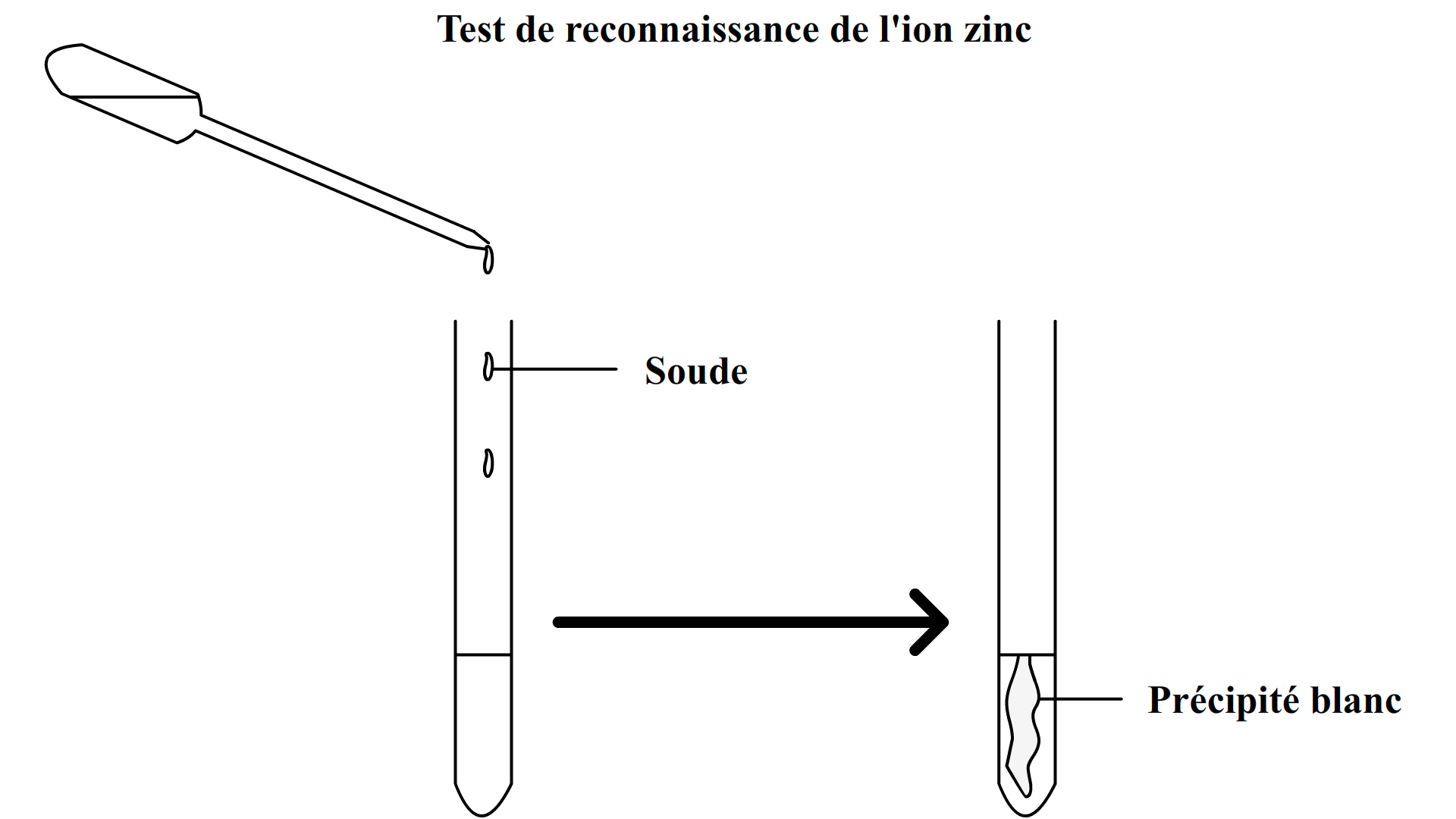
Zinc Ion Test . Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution of the ammonia complex. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. Zinc is probably one of the only elements that form a white precipitate with sulfide ions.
from physique-chimie-college.fr
Zinc forms one of the few insoluble white sulfides. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. It is precipitated from a solution of the ammonia complex. Zinc is probably one of the only elements that form a white precipitate with sulfide ions.
Transformation chimique solution de sulfate de cuivre et poudre de
Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc forms one of the few insoluble white sulfides. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution of the ammonia complex.
From www.youtube.com
Test for zinc ions using sodium hydroxide GCSE YouTube Zinc Ion Test Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc is probably one of the only elements that form a white precipitate with sulfide ions. It is precipitated from a solution of the ammonia complex. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc (zn²⁺) confirmatory tests should be. Zinc Ion Test.
From essopenarchive.org
Enhanced ZincIon Batteries through the Coating of Surface Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution of the ammonia complex. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. \[\ce{zn^{2+}(aq) +. Zinc Ion Test.
From www.lelivrescolaire.fr
Comment détecter des ions ? Lelivrescolaire.fr Zinc Ion Test Zinc forms one of the few insoluble white sulfides. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. It is precipitated from a solution of the ammonia complex. Zinc is probably one of the only elements that form a white precipitate with. Zinc Ion Test.
From pubs.rsc.org
Fundamentals and perspectives in developing zincion battery Zinc Ion Test It is precipitated from a solution of the ammonia complex. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc is probably one of the only elements that form a white precipitate with sulfide ions.. Zinc Ion Test.
From www.youtube.com
HSC Chemistry 1st Paper PracticalZinc Ion Test YouTube Zinc Ion Test Zinc forms one of the few insoluble white sulfides. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. It is. Zinc Ion Test.
From www.researchgate.net
The ion exchange isotherms for zinc ions in NaAc/ZnAc 2 solution at Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. It is precipitated from a solution of the ammonia complex. Zinc (zn²⁺) confirmatory tests should be performed on. Zinc Ion Test.
From www.youtube.com
How to find Protons & Electrons for the Zn 2+ (Zinc ion) YouTube Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. Zinc is probably one of the only elements that form a white precipitate. Zinc Ion Test.
From www.spc10.fr
Les tests d’identification SPC10 Zinc Ion Test Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc forms one of the few insoluble white sulfides. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking. Zinc Ion Test.
From www.researchgate.net
Comparison of selectivity for zinc ions from binary solutions of Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution. Zinc Ion Test.
From passive-components.eu
Researchers Developed Ti/Si/C Composite Zincion Supercapacitor Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions. Zinc Ion Test.
From www.aliexpress.com
Lu Heng biological zinc rapid detection colorimetric tube zinc ion Zinc Ion Test Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. It is precipitated from a solution of the ammonia complex. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order. Zinc Ion Test.
From www.degruyter.com
The progress of cathode materials in aqueous zincion batteries Zinc Ion Test \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. It is precipitated from a solution of the ammonia complex. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to. Zinc Ion Test.
From www.youtube.com
Cation Test Zinc Ions YouTube Zinc Ion Test Zinc forms one of the few insoluble white sulfides. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc (zn²⁺) confirmatory tests should be performed. Zinc Ion Test.
From www.science.org
A rechargeable zincair battery based on zinc peroxide chemistry Science Zinc Ion Test Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution of the ammonia complex. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few. Zinc Ion Test.
From www.youtube.com
Ionic Charge for Zinc (Zn) YouTube Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. It is precipitated from a solution of the ammonia complex. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc forms. Zinc Ion Test.
From www.researchgate.net
Electrochemical performance of the Zn‐ion battery. a) EIS profile Zinc Ion Test It is precipitated from a solution of the ammonia complex. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc (zn²⁺). Zinc Ion Test.
From www.youtube.com
Test for Zinc ion with Aqueous Ammonia YouTube Zinc Ion Test \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc forms one of the few. Zinc Ion Test.
From smartmouth.com
How SmartMouth™ Works The Power of Zinc Ion Technology Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc forms one of the few insoluble white sulfides. It is precipitated from a solution of the ammonia complex. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc (zn²⁺). Zinc Ion Test.
From www.researchgate.net
Zinc ionsupported autocleavage of HS in Gpc1 monitored by Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc forms one of the few insoluble white sulfides. It is precipitated from a solution of the ammonia complex. Zinc(ii) ion reacts. Zinc Ion Test.
From www.youtube.com
QUALITATIVE ANALYSIS 2. Test for Zinc II ion. Confirmatory Test for Zn² Zinc Ion Test It is precipitated from a solution of the ammonia complex. Zinc forms one of the few insoluble white sulfides. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc(ii) ion. Zinc Ion Test.
From www.youtube.com
Cation Test Zinc ion with Sodium Hydroxide YouTube Zinc Ion Test \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. Zinc is probably one of the only. Zinc Ion Test.
From 2012books.lardbucket.org
Electrochemistry Zinc Ion Test \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc forms one of the few insoluble white sulfides. It is precipitated from a solution of the ammonia complex. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water. Zinc Ion Test.
From www.researchgate.net
Zn/Zn symmetric cell test results at a current density of 0.5 mA/cm 2 Zinc Ion Test It is precipitated from a solution of the ammonia complex. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc. Zinc Ion Test.
From byjus.com
the zinc blended structure the coordination number of Zn+2 ion is Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. It is precipitated from a solution of the ammonia complex. Zinc forms one of the few insoluble white sulfides. Zinc(ii) ion reacts with aqueous ammonia to precipitate. Zinc Ion Test.
From pubs.acs.org
NiContaining Electrolytes for Superior ZincIon Aqueous Batteries with Zinc Ion Test Zinc is probably one of the only elements that form a white precipitate with sulfide ions. It is precipitated from a solution of the ammonia complex. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc (zn²⁺) confirmatory tests should be. Zinc Ion Test.
From www.researchgate.net
(a) Chargedischarge curves of a zincair battery, with air electrodes Zinc Ion Test Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc forms one of the few insoluble white sulfides. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking. Zinc Ion Test.
From pubs.acs.org
In Situ Construction of Protective Films on Zn Metal Anodes via Natural Zinc Ion Test Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc forms one of the few insoluble white sulfides. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution of the ammonia complex. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your. Zinc Ion Test.
From www.youtube.com
Tests for zinc Ion MeitY OLabs YouTube Zinc Ion Test Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions. Zinc Ion Test.
From www.slideshare.net
The periodic table and identification of ions Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. It is precipitated from a solution of the ammonia complex. Zinc is probably one of the. Zinc Ion Test.
From www.researchgate.net
Relationship of zinc ion availability (ZIA) values and reduction in Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>.. Zinc Ion Test.
From sciencesphysiques.e-monsite.com
Test des ions Zinc Ion Test Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. It is precipitated from a solution of the ammonia complex. Zinc forms one of the few insoluble. Zinc Ion Test.
From www.shutterstock.com
A Collection Of Ion Flame Tests Zinc, Potassium,Strontium ,Sodium,And Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: It is precipitated from a solution of the ammonia complex. Zinc forms one of the few insoluble white sulfides. Zinc is probably one of the only elements that form a white precipitate. Zinc Ion Test.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Ion Test It is precipitated from a solution of the ammonia complex. An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>.. Zinc Ion Test.
From www.techbriefs.com
A Novel Strategy to Stabilize ZincIon Batteries Tech Briefs Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc is probably one of the only elements that form a white precipitate with sulfide ions. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>.. Zinc Ion Test.
From physique-chimie-college.fr
Transformation chimique solution de sulfate de cuivre et poudre de Zinc Ion Test An electrochemical method for detecting the presence of zinc (zn2+) ions in drinking water was developed using. Zinc forms one of the few insoluble white sulfides. Zinc (zn²⁺) confirmatory tests should be performed on separate solutions of some of your ions, in order to see what these. Zinc is probably one of the only elements that form a white precipitate. Zinc Ion Test.