For Mixing Of Liquids Benzene And Toluene Entropy Change Is . We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Molecules look alike to one another. Mixture is an ideal solution.
from www.numerade.com
If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Molecules look alike to one another. Mixture is an ideal solution. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids.
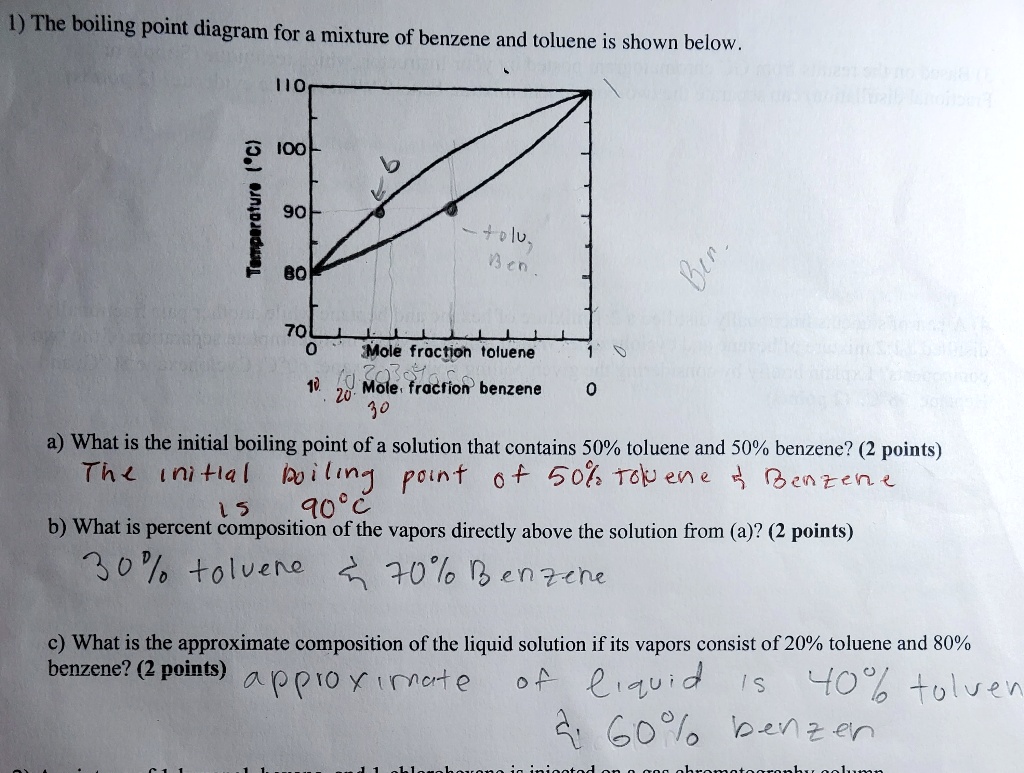
SOLVED The boiling point diagram for a mixture of benzene and toluene
For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Molecules look alike to one another. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. Mixture is an ideal solution.
From www.chegg.com
Solved Q1 A liquid mixture of benzene toluene is being For Mixing Of Liquids Benzene And Toluene Entropy Change Is Molecules look alike to one another. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. Mixture is an ideal solution. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike,. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.toppr.com
Benzene and toluene form an ideal solution. A liquid solution is formed For Mixing Of Liquids Benzene And Toluene Entropy Change Is We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved 2. The temperature phase diagram for benzene/toluene For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved 10) A mixture of toluene and benzene For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From learncheme.com
LearnChemE For Mixing Of Liquids Benzene And Toluene Entropy Change Is Mixture is an ideal solution. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From chempedia.info
Toluenebenzene mixtures Big Chemical Encyclopedia For Mixing Of Liquids Benzene And Toluene Entropy Change Is Molecules look alike to one another. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Since there are only half as many benzene molecules in the mixture as in. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.toppr.com
The enthalpy of vaporisation of benzene is +35.3 kJ/mol its boiling For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From byjus.com
Benzene and toluene form nearly ideal solutions. At T = 300 K, the For Mixing Of Liquids Benzene And Toluene Entropy Change Is Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From chempedia.info
Benzene/toluene mixtures, phase diagram Big Chemical Encyclopedia For Mixing Of Liquids Benzene And Toluene Entropy Change Is Molecules look alike to one another. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. We can use the experimental result that (for similar liquids) the mole fraction of. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.numerade.com
SOLVED The boiling point diagram for a mixture of benzene and toluene For Mixing Of Liquids Benzene And Toluene Entropy Change Is Molecules look alike to one another. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. If we use the initial volumes v a and v b for the initial volumes of gases a and b,. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved 7.55. A liquid mixture of benzene and toluene is to For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Molecules look alike to one another. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved A mixture of benzene and toluene is separated into For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Mixture is an ideal solution. Molecules look alike to one another. We can use the experimental result that (for similar. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved A liquid mixture of benzene (B), toluene (T) and For Mixing Of Liquids Benzene And Toluene Entropy Change Is Mixture is an ideal solution. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Molecules look alike to one another. Since there are only half as many benzene molecules. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved A mixture of benzene (MW = 78) and toluene (MW = 92) For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.numerade.com
SOLVED Refer to the vaporliquid composition curve for a benzene For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike,. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.numerade.com
SOLVED The boiling point diagram for a mixture of benzene and toluene For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Mixture is an ideal solution. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.numerade.com
SOLVED A binary mixture of benzene and toluene is to be separated in a For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Molecules look alike to one another. Mixture is an ideal solution. Since there are only half as many benzene molecules in the mixture as in pure. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.youtube.com
11.02 Entropy Change Upon Mixing YouTube For Mixing Of Liquids Benzene And Toluene Entropy Change Is We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Mixture is an ideal solution. Molecules look alike to one another. It is easy to explain this behavior if we assume that because benzene and toluene. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved Question 2 Benzene and Toluene form an ideal liquid For Mixing Of Liquids Benzene And Toluene Entropy Change Is Mixture is an ideal solution. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved An equimolar liquid mixture of benzene and toluene is For Mixing Of Liquids Benzene And Toluene Entropy Change Is Molecules look alike to one another. Mixture is an ideal solution. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. We can use the experimental result that (for similar. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From chempedia.info
Toluenebenzene mixtures Big Chemical Encyclopedia For Mixing Of Liquids Benzene And Toluene Entropy Change Is We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Mixture is an ideal solution. Molecules look alike to one another. Since there are only half as many benzene molecules in the mixture as in pure. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved Vaporliquid equilibrium data for are For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Molecules look alike to one another. Since there are only half as many benzene molecules in the mixture as in. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From learncheme.com
LearnChemE For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.numerade.com
SOLVED A liquid stream containing equimolar amounts of benzene and For Mixing Of Liquids Benzene And Toluene Entropy Change Is Mixture is an ideal solution. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved s2. A mixture of toluene and benzene For Mixing Of Liquids Benzene And Toluene Entropy Change Is We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Mixture is an ideal solution. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved 1. A liquid mixture of Benzene (C6H6) and Toluene For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved QUESTION 3 A liquid mixture of benzene toluene is For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Molecules look alike to one another. Mixture is an ideal solution. We can use the experimental result that (for similar. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved An equimolar mixture of benzene and toluene is For Mixing Of Liquids Benzene And Toluene Entropy Change Is Molecules look alike to one another. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Mixture is an ideal solution. Since there are only half as many benzene molecules. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
A liquid mixture of benzene toluene is to be For Mixing Of Liquids Benzene And Toluene Entropy Change Is Mixture is an ideal solution. Molecules look alike to one another. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. It is easy to explain this behavior if we assume that because benzene and toluene. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.coursehero.com
[Solved] An equimolar liquid mixture of benzene and toluene is For Mixing Of Liquids Benzene And Toluene Entropy Change Is Mixture is an ideal solution. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Since there are only half as many benzene molecules in the mixture as in pure. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From brainly.com
An ideal liquid mixture of benzene and toluene (50 by volume each) is For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Molecules look alike to one another. It is easy to explain this behavior if we assume that because benzene and. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.numerade.com
SOLVED How will the mole fractions of benzene and tolucne in the For Mixing Of Liquids Benzene And Toluene Entropy Change Is It is easy to explain this behavior if we assume that because benzene and toluene molecules are so nearly alike, they behave the same way in solution as they do in the pure liquids. We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.coursehero.com
[Solved] A liquid mixture of benzene and toluene contains 55.0 benzene For Mixing Of Liquids Benzene And Toluene Entropy Change Is We can use the experimental result that (for similar liquids) the mole fraction of the substance a in the mixture is approximately pa/pa *, or the ratio of the partial vapor pressures of each. Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From www.chegg.com
Solved 7.55. A liquid mixture of benzene and toluene is to For Mixing Of Liquids Benzene And Toluene Entropy Change Is Since there are only half as many benzene molecules in the mixture as in pure benzene, the rate at which benzene molecules escape from the surface of the solution. If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.
From pdfprof.com
an ideal solution of benzene and toluene For Mixing Of Liquids Benzene And Toluene Entropy Change Is If we use the initial volumes v a and v b for the initial volumes of gases a and b, the total volume after mixing is \(v_a + v_b\), and the total entropy change is \[\delta s_{mix} = n_ar \ln. Mixture is an ideal solution. We can use the experimental result that (for similar liquids) the mole fraction of the. For Mixing Of Liquids Benzene And Toluene Entropy Change Is.