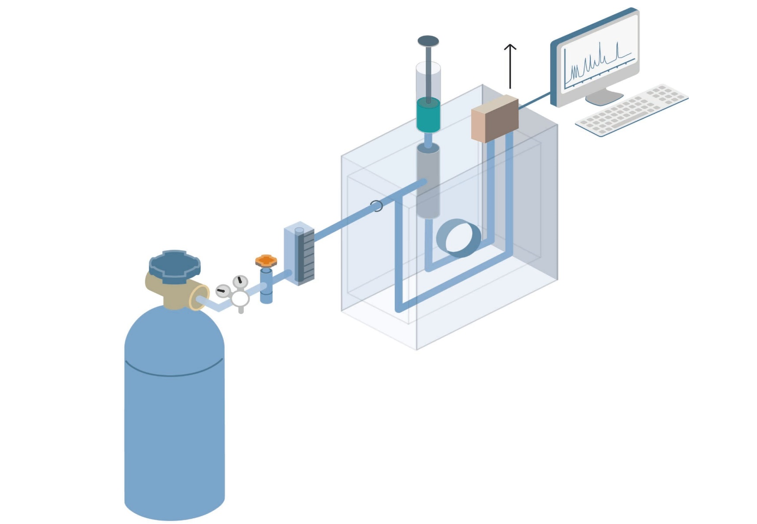
Gas Chromatography Boiling Point . Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. How fast a particular compound travels. The lower the boiling point is, the. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. The boiling point of a compound is often related to its polarity (see also polarity chapter). The boiling point of the compound. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the.
from www.creative-proteomics.com
Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of the compound. The lower the boiling point is, the. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. How fast a particular compound travels. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. The boiling point of a compound is often related to its polarity (see also polarity chapter).
Introduction to Gas Chromatography—Principles, Characteristics, and
Gas Chromatography Boiling Point How fast a particular compound travels. The lower the boiling point is, the. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. How fast a particular compound travels. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. The boiling point of the compound. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of a compound is often related to its polarity (see also polarity chapter). Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte.
From present5.com
Lecture X Gas Chromatography Outline èGC Theory Gas Chromatography Boiling Point Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical. Gas Chromatography Boiling Point.
From slideplayer.com
CHROMATOGRAPHY. ppt download Gas Chromatography Boiling Point Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Gas chromatography is one of the sole forms of chromatography that does not utilize. Gas Chromatography Boiling Point.
From www.priyamstudycentre.com
Gas Chromatography Machine, Instrumentation, Applications Gas Chromatography Boiling Point A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. How fast a particular compound travels. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. The boiling point of. Gas Chromatography Boiling Point.
From lab-training.com
Gas Chromatography Principles, Types and Working Gas Chromatography Boiling Point The boiling point of the compound. How fast a particular compound travels. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of a compound is often related to its polarity (see also polarity chapter). A compound which boils at a temperature higher than the column temperature is. Gas Chromatography Boiling Point.
From www.slideshare.net
Gas Chromatography Fall 09 Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical. Gas Chromatography Boiling Point.
From www.studocu.com
Boiling Point Lab report Boiling Point, Refractive Index, and Gas Chromatography Boiling Point Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Ch 21 Principles of Chromatography and Mass Spectrometry Ch 22 Gas Chromatography Boiling Point Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. The lower the boiling point is, the. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography (gc) is a common type of chromatography in which the. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT GAS CHROMATOGRAPHY PowerPoint Presentation, free download ID Gas Chromatography Boiling Point Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. A compound which boils at a temperature higher than the column temperature is going to. Gas Chromatography Boiling Point.
From www.creative-proteomics.com
Introduction to Gas Chromatography—Principles, Characteristics, and Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. The boiling point of the compound. Gas chromatography is akin to. Gas Chromatography Boiling Point.
From mavink.com
Gas Chromatography Labelled Diagram Gas Chromatography Boiling Point A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. The boiling point of the compound. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. Gas chromatography is one. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT GAS CHROMATOGRAPHY (GC) PowerPoint Presentation, free download Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. The boiling point of the compound. The boiling point of a compound is often related to its polarity (see also polarity chapter). How fast a particular compound travels. Gas chromatography (gc) is a common type of chromatography in. Gas Chromatography Boiling Point.
From instrumentationtools.com
What is Gas Chromatography? Gas Chromatography Boiling Point Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. The lower the boiling point is, the. The boiling point of a compound is often related to its polarity (see also polarity chapter). How fast a particular compound travels. Gas chromatography is one of the sole forms of. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point The lower the boiling point is, the. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. How fast a particular compound travels. Gas chromatography. Gas Chromatography Boiling Point.
From www.slideshare.net
gas chromatography (GC) Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. The boiling point of the compound. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Since the process of dissolving. Gas Chromatography Boiling Point.
From www.thoughtco.com
Gas Chromatography What It Is and How It Works Gas Chromatography Boiling Point The boiling point of the compound. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Gas chromatography is akin to. Gas Chromatography Boiling Point.
From www.rdworldonline.com
What is Gas Chromatography Research & Development World Gas Chromatography Boiling Point Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. The lower the boiling point is, the. The boiling point of a compound is often related to its polarity. Gas Chromatography Boiling Point.
From www.youtube.com
Gas chromatography basic theory, principles and application. YouTube Gas Chromatography Boiling Point Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of a compound is often related to its polarity (see also polarity chapter). Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. How fast a. Gas Chromatography Boiling Point.
From www.youtube.com
Gas chromatography principle and instrumentation YouTube Gas Chromatography Boiling Point Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. The lower the boiling point is, the. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. How fast a particular. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. The lower the boiling point is, the. How fast a particular compound travels. A compound which. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point The boiling point of the compound. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Since the process of. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. The lower the boiling point is, the. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography (gc) is a common type. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Ch 21 Principles of Chromatography and Mass Spectrometry Ch 22 Gas Chromatography Boiling Point A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography is one of the sole forms of chromatography that does not utilize. Gas Chromatography Boiling Point.
From www.slideshare.net
Gas Chromatography Gas Chromatography Boiling Point Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. A compound which boils at a temperature higher than the column temperature is going to. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT An Introduction to Chromatography PowerPoint Presentation, free Gas Chromatography Boiling Point How fast a particular compound travels. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of a compound is often related to its polarity (see. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT GAS CHROMATOGRAPHY PowerPoint Presentation, free download ID Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. The lower the boiling point is, the. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Since the process of. Gas Chromatography Boiling Point.
From dokumen.tips
(PPT) 1 Chapter 24 GC Gas Chromatography. 2 GC Mechanism of separation Gas Chromatography Boiling Point The boiling point of the compound. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of a compound is often related to its polarity (see also polarity chapter). Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting. Gas Chromatography Boiling Point.
From dokumen.tips
(PPTX) Gas Chromatography. Gas Chromatography (GC) This method depends Gas Chromatography Boiling Point The boiling point of a compound is often related to its polarity (see also polarity chapter). A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. How fast a particular compound travels. Gas chromatography (gc) is a common type of chromatography in which. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point The boiling point of a compound is often related to its polarity (see also polarity chapter). How fast a particular compound travels. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical. Gas Chromatography Boiling Point.
From mungfali.com
Gas Chromatography Diagram Gas Chromatography Boiling Point How fast a particular compound travels. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Gas chromatography is akin to. Gas Chromatography Boiling Point.
From chem.libretexts.org
12.4 Gas Chromatography Chemistry LibreTexts Gas Chromatography Boiling Point The boiling point of a compound is often related to its polarity (see also polarity chapter). Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating. Gas Chromatography Boiling Point.
From www.numerade.com
SOLVED A chromatogram is shown as followed. (a) If this is a gas Gas Chromatography Boiling Point The lower the boiling point is, the. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. Gas chromatography (gc) is a common type of chromatography in which the mobile phase is gas used in analytical chemistry for separating and. The boiling point of the compound. Since the process of dissolving. Gas Chromatography Boiling Point.
From chem.libretexts.org
12.4 Gas Chromatography Chemistry LibreTexts Gas Chromatography Boiling Point The boiling point of a compound is often related to its polarity (see also polarity chapter). The boiling point of the compound. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. How fast a particular compound travels. A compound which boils at a temperature higher than the. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography Acetates PowerPoint Presentation, free Gas Chromatography Boiling Point The boiling point of the compound. Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. The lower the boiling point is, the. The boiling point of a compound is often related to its polarity (see also polarity chapter). How fast a particular compound travels. A compound which. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point A compound which boils at a temperature higher than the column temperature is going to spend nearly all of its time condensed as a liquid at the. Since the process of dissolving and vaporizing the sample in the stationary phase depends on the physicochemical properties such as the boiling. Gas chromatography (gc) is a common type of chromatography in which. Gas Chromatography Boiling Point.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID Gas Chromatography Boiling Point Gas chromatography is one of the sole forms of chromatography that does not utilize the mobile phase for interacting with the analyte. Gas chromatography is akin to fractional distillation, in which the more volatile components of a mixture are collected first. The boiling point of the compound. Gas chromatography (gc) is a common type of chromatography in which the mobile. Gas Chromatography Boiling Point.