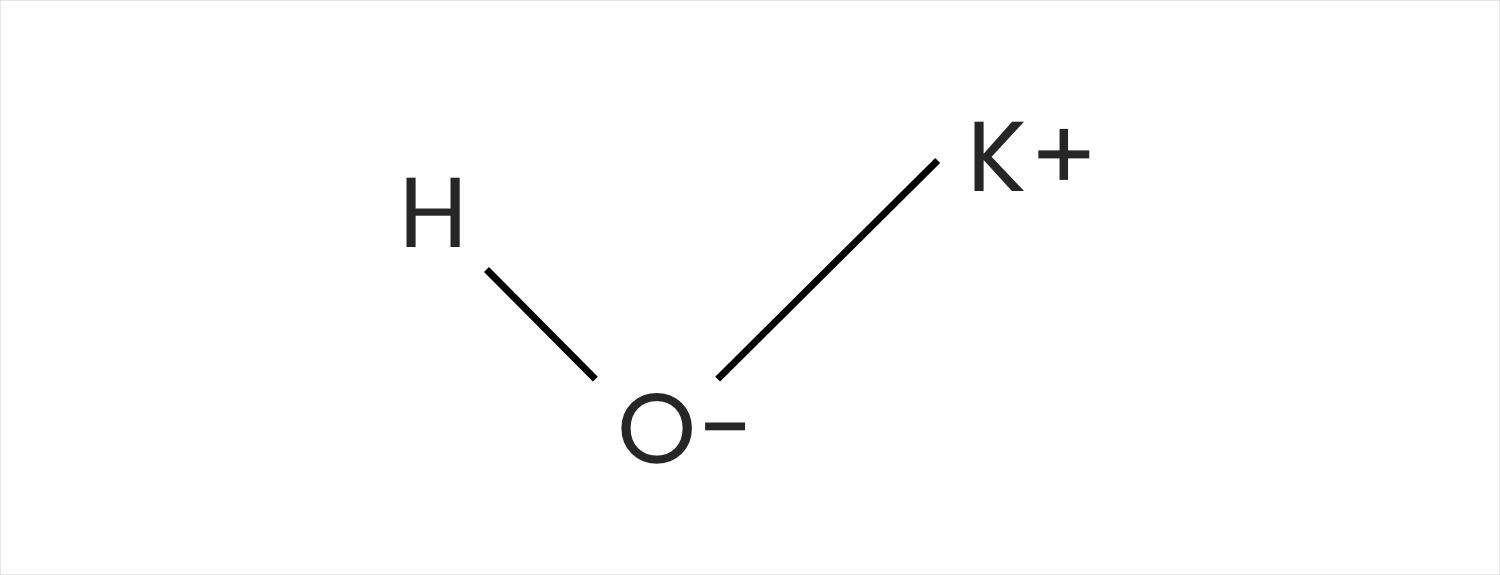
Why Is Potassium Hydroxide Used To Dissolve The Aluminum . aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. If there are any white flecks left in the solution after the addition. aluminum hydroxide becomes aluminum sulfate and dissolves. used for the advanced topics in chemistry class in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. To prepare a potassium alum salt, and determine.
from thedermreview.com
in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. To prepare a potassium alum salt, and determine. aluminum hydroxide becomes aluminum sulfate and dissolves. If there are any white flecks left in the solution after the addition. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. used for the advanced topics in chemistry class aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts.
Potassium Hydroxide Why Is Potassium Hydroxide Used? And Do You Need To Be Careful With This
Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. If there are any white flecks left in the solution after the addition. used for the advanced topics in chemistry class aluminum hydroxide becomes aluminum sulfate and dissolves. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. To prepare a potassium alum salt, and determine. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to.
From www.youtube.com
Equation for Potassium Chloride Dissolving in Water ( KCl + H2O) YouTube Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. used for the advanced topics in chemistry class aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.slideshare.net
Chapter 14.1 Compounds in Aqueous Solutions Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. If there are any white flecks. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From blog.agchemigroup.eu
The Usefulness of Potassium Hydroxide Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. aluminum hydroxide becomes aluminum sulfate and dissolves. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From proper-cooking.info
Potassium Hydroxide Chemical Structure Why Is Potassium Hydroxide Used To Dissolve The Aluminum If there are any white flecks left in the solution after the addition. aluminum hydroxide becomes aluminum sulfate and dissolves. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. aluminum is considered a reactive metal, but because its surface is usually protected by a thin. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From formulasense.com
What is Potassium Hydroxide? — Formula Sense Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum hydroxide becomes aluminum sulfate and dissolves. To prepare a potassium alum salt, and determine. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.youtube.com
How to Balance Al2(SO4)3 + KOH = Al(OH)3 + K2SO4 (Aluminum sulfate + Potassium hydroxide) YouTube Why Is Potassium Hydroxide Used To Dissolve The Aluminum used for the advanced topics in chemistry class aluminum hydroxide becomes aluminum sulfate and dissolves. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.pw.live
Potassium Hydroxide Formula, Structure, Properties, Uses Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. in the first chemical step of the experiment, you will add a. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.chemsupplies.com.au
Potassium Hydroxide KOH Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. used for the advanced topics in. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.doubtnut.com
Why is a solution of potassium hydroxide used to absorb carbon dioxide evolved during the Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum hydroxide becomes aluminum sulfate and dissolves. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. aluminum is considered a reactive. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.chegg.com
Solved Aluminum dissolves in HCl according to the equation Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum hydroxide becomes aluminum sulfate and dissolves. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. To prepare a potassium alum salt, and determine. used for the advanced topics in chemistry class aluminum is considered a reactive metal, but because its surface. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.slideserve.com
PPT HelloOo…. PowerPoint Presentation ID2068170 Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. If there are any white flecks left in the solution after the addition. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. used for. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.slideserve.com
PPT Aluminum hydroxide PowerPoint Presentation, free download ID2372960 Why Is Potassium Hydroxide Used To Dissolve The Aluminum If there are any white flecks left in the solution after the addition. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. . Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From armsingle10.pythonanywhere.com
Divine Potassium Hydroxide Plus Water Fundamental And Derived Quantities Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum hydroxide becomes aluminum sulfate and dissolves. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. used for the advanced topics in chemistry. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.numerade.com
SOLVED You can dissolve an aluminum soft drink can in an aqueous base such as potassium Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. If there are any white flecks left in the solution after the addition. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. the. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.pinterest.com
Difference Between Potassium Hydroxide and Sodium Hydroxide Definition, Properties Why Is Potassium Hydroxide Used To Dissolve The Aluminum If there are any white flecks left in the solution after the addition. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. aluminum hydroxide becomes aluminum sulfate and dissolves. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.youtube.com
39 Uses of Potassium Hydroxide YouTube Why Is Potassium Hydroxide Used To Dissolve The Aluminum the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. aluminum hydroxide becomes aluminum sulfate and dissolves. To prepare a potassium alum salt,. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.chemicals.co.uk
Potassium Hydroxide Uses, Formula, Dangers & More Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. If there are any white flecks left in the solution after the addition. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. . Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104 Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. aluminum hydroxide becomes aluminum sulfate and dissolves. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From qbotchemi.com
Screenshot_2 Why Is Potassium Hydroxide Used To Dissolve The Aluminum used for the advanced topics in chemistry class in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. If there are any white flecks left in the solution after the addition. To prepare a potassium alum salt, and determine. aluminum is considered a reactive metal, but. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.numerade.com
SOLVED In the original reaction, aluminum dissolved In potassium hydroxide solutian What kind Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. aluminum hydroxide becomes aluminum sulfate and dissolves. To prepare a potassium alum salt, and determine. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From thedermreview.com
Potassium Hydroxide Why Is Potassium Hydroxide Used? And Do You Need To Be Careful With This Why Is Potassium Hydroxide Used To Dissolve The Aluminum the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. aluminum hydroxide becomes aluminum sulfate and dissolves. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. used for the advanced. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From testbook.com
Potassium Hydroxide Learn Definition, Symbol, Preparation here Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. To prepare a potassium alum salt, and determine. If there are any white flecks left in the solution after the addition. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From myloview.com
Potassium hydroxide chemical structure. vector illustration. posters for the wall • posters Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. To prepare a potassium alum salt, and determine. used for the advanced topics in chemistry class If there are any white flecks left in the solution after the addition. in the first chemical step of the. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From pharmabeej.com
What Is The Molecular Formula Of Potassium Hydroxide? Pharmabeej Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. in the experiment, recycled aluminum can is used to react with potassium hydroxide. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.numerade.com
SOLVED Consider the reaction when aqueous solutions of aluminum bromide and potassium hydroxide Why Is Potassium Hydroxide Used To Dissolve The Aluminum If there are any white flecks left in the solution after the addition. aluminum hydroxide becomes aluminum sulfate and dissolves. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. used for the advanced topics in chemistry class aluminum is considered a reactive. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From socratic.org
Question 300fd Socratic Why Is Potassium Hydroxide Used To Dissolve The Aluminum aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. used for the advanced topics in chemistry class To prepare a potassium alum salt, and determine. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.museoinclusivo.com
What is the Formula for Aluminum Hydroxide? Exploring its Components and Chemistry Aluminum Why Is Potassium Hydroxide Used To Dissolve The Aluminum used for the advanced topics in chemistry class aluminum hydroxide becomes aluminum sulfate and dissolves. If there are any white flecks left in the solution after the addition. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. To prepare a potassium alum salt,. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.youtube.com
Dissolving Aluminum in Potassium Hydroxide YouTube Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. used for the advanced topics in chemistry class in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. aluminum is considered a reactive metal,. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.youtube.com
Potassium Hydroxide Reacting with Aluminum YouTube Why Is Potassium Hydroxide Used To Dissolve The Aluminum used for the advanced topics in chemistry class If there are any white flecks left in the solution after the addition. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. aluminum hydroxide becomes aluminum sulfate and dissolves. To prepare a potassium alum salt, and determine.. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From sciencestruck.com
39 Uses of Potassium Hydroxide You Probably Never Imagined Science Struck Why Is Potassium Hydroxide Used To Dissolve The Aluminum used for the advanced topics in chemistry class aluminum hydroxide becomes aluminum sulfate and dissolves. in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. To prepare a potassium alum salt, and determine. If there are any white flecks left in the solution after the addition.. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From qbotchemi.com
Screenshot_2 Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. aluminum hydroxide becomes aluminum sulfate and dissolves. To prepare a potassium alum salt, and determine. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From issuu.com
What are the Properties and Applications of Potassium Hydroxide? by Palvi Fze Issuu Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. in the first chemical step. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From library.fiveable.me
The Dissolution Process Intro to Chemistry Class Notes Fiveable Why Is Potassium Hydroxide Used To Dissolve The Aluminum the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. aluminum is considered a reactive metal, but because its surface is usually protected by a thin film of aluminum oxide, it reacts. in the first chemical step of the experiment, you will add a. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.slideserve.com
PPT Palvi FZE What are the Properties and Applications of Potassium Hydroxide_ (1 Why Is Potassium Hydroxide Used To Dissolve The Aluminum To prepare a potassium alum salt, and determine. the first step in this synthesis, which you will perform during week 1, is to react metallic aluminum with a concentrated solution of. used for the advanced topics in chemistry class If there are any white flecks left in the solution after the addition. aluminum is considered a reactive. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts..? Exothermic Reaction YouTube Why Is Potassium Hydroxide Used To Dissolve The Aluminum in the first chemical step of the experiment, you will add a strong base, potassium hydroxide (koh), to your aluminum foil. in the experiment, recycled aluminum can is used to react with potassium hydroxide and sulfuric acid in sequence in order to. the first step in this synthesis, which you will perform during week 1, is to. Why Is Potassium Hydroxide Used To Dissolve The Aluminum.