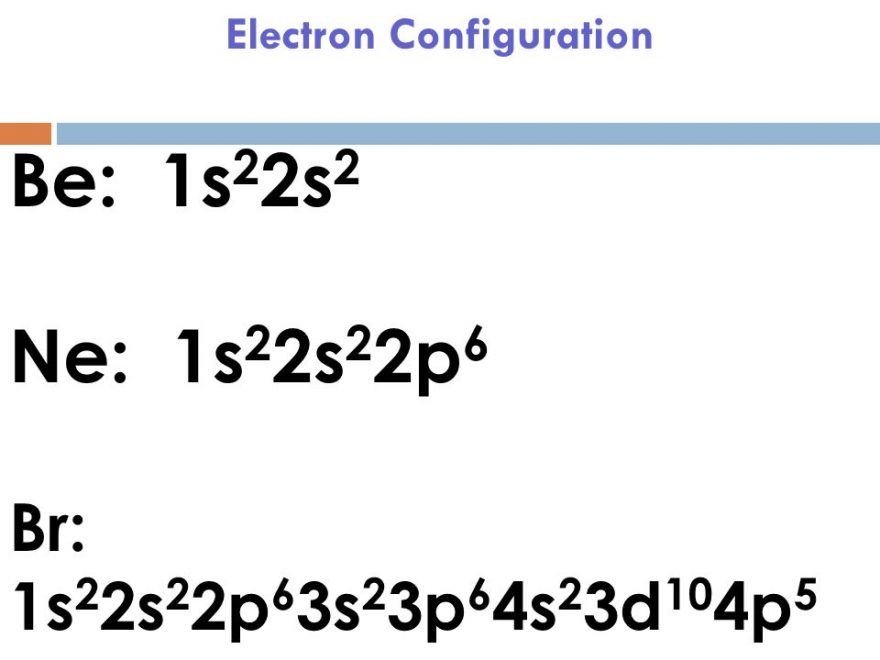
Bromine Valence Number . There are two ways to find the number of valence electrons in bromine (br). In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). Please read the full article below for more information. Let’s break down each method in. We can write the valence electrons of bromine using two different methods: The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Also, we will provide the pictures of the same. What is the valency of bromine? #1 using periodic table #2 using electron configuration. Bromine has an electron configuration of #1s^2 2s^2 2p^6. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Valence electrons found in the s and p orbitals of the highest energy. The first is to use the.
from periodictable.me
Let’s break down each method in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. #1 using periodic table #2 using electron configuration. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. There are two ways to find the number of valence electrons in bromine (br). We can write the valence electrons of bromine using two different methods: The first is to use the. What is the valency of bromine? Bromine has an electron configuration of #1s^2 2s^2 2p^6. Also, we will provide the pictures of the same.
Bromine Electron Configuration (Br) with Orbital Diagram
Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. There are two ways to find the number of valence electrons in bromine (br). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). What is the valency of bromine? Bromine has an electron configuration of #1s^2 2s^2 2p^6. We can write the valence electrons of bromine using two different methods: Valence electrons found in the s and p orbitals of the highest energy. Also, we will provide the pictures of the same. #1 using periodic table #2 using electron configuration. Please read the full article below for more information. The first is to use the. Let’s break down each method in.
From www.anyrgb.com
Valence electron, Electron shell, Bromine, bromide, Electron Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Please read the. Bromine Valence Number.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Valence Number Please read the full article below for more information. #1 using periodic table #2 using electron configuration. Also, we will provide the pictures of the same. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The ability of one atom of an element to join another atom. Bromine Valence Number.
From www.youtube.com
How many valence electrons does bromine have? YouTube Bromine Valence Number In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine has an electron configuration of #1s^2 2s^2 2p^6. There are two ways to find the number of valence electrons in bromine (br). Please read the full article below for more information. #1 using periodic table #2 using. Bromine Valence Number.
From www.youtube.com
How to Find the Valence Electrons for Bromine (Br) YouTube Bromine Valence Number 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Please read the full article below for more information. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell. Bromine Valence Number.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Valence Number 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The first is to use the. #1 using periodic table #2 using electron configuration. Also, we will provide the pictures of the same. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an. Bromine Valence Number.
From byjus.com
What is the oxidation number of bromine in sequence in Br3O8? Bromine Valence Number Bromine has an electron configuration of #1s^2 2s^2 2p^6. #1 using periodic table #2 using electron configuration. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.. Bromine Valence Number.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Valence Number 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Valence electrons found in the s and p orbitals of the highest energy. What is the valency of bromine? Also, we will provide the pictures of the same. In this article today we are going to tell you about. Bromine Valence Number.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Valence Number #1 using periodic table #2 using electron configuration. Bromine has an electron configuration of #1s^2 2s^2 2p^6. What is the valency of bromine? Valence electrons found in the s and p orbitals of the highest energy. Let’s break down each method in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Bromine Valence Number.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). Let’s break down each method in. Valence electrons found in the s and p orbitals of the highest energy. The first is to use the. The ability of one atom of an element to join another atom during the formation of a molecule is called valency. Bromine Valence Number.
From www.shutterstock.com
Bromine Element Mendeleev Periodic Table Magnified Stock Photo Bromine Valence Number The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. What is the valency of bromine? #1 using periodic table #2 using electron configuration. In this article today we are going to tell you about the electron configuration. Bromine Valence Number.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Valence Number Also, we will provide the pictures of the same. What is the valency of bromine? There are two ways to find the number of valence electrons in bromine (br). In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. #1 using periodic table #2 using electron configuration. We. Bromine Valence Number.
From www.youtube.com
Electron Configuration of Bromine Br Lesson YouTube Bromine Valence Number Valence electrons found in the s and p orbitals of the highest energy. Also, we will provide the pictures of the same. #1 using periodic table #2 using electron configuration. The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). The first is to use the. The total. Bromine Valence Number.
From proper-cooking.info
Bromine Valence Electrons Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). Please read the full article below for more information. What is the valency of bromine? Let’s break down each method in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. We can write the. Bromine Valence Number.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Bromine Valence Number What is the valency of bromine? The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Bromine has an electron configuration of #1s^2 2s^2 2p^6. In this article today we are going to tell you about the electron. Bromine Valence Number.
From www.vectorstock.com
Symbol and electron diagram for bromine Royalty Free Vector Bromine Valence Number In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. What is the valency of bromine?. Bromine Valence Number.
From ar.inspiredpencil.com
Bromine Valence Electrons Bromine Valence Number The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Bromine has an electron configuration of #1s^2 2s^2 2p^6. #1 using periodic table #2 using electron configuration. Please read the full article below for more information. In this. Bromine Valence Number.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Bromine Valence Number The first is to use the. There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. What is the valency of bromine? In this. Bromine Valence Number.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Valence Number Bromine has an electron configuration of #1s^2 2s^2 2p^6. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. #1 using periodic table #2 using electron configuration. Valence electrons found in the s and p orbitals of the. Bromine Valence Number.
From brainly.in
bromine valence electrons, Brainly.in Bromine Valence Number Valence electrons found in the s and p orbitals of the highest energy. #1 using periodic table #2 using electron configuration. There are two ways to find the number of valence electrons in bromine (br). We can write the valence electrons of bromine using two different methods: The ability of one atom of an element to join another atom during. Bromine Valence Number.
From www.chemistrylearner.com
Bromine Facts, Symbol, Discovery, Properties, Uses Bromine Valence Number Valence electrons found in the s and p orbitals of the highest energy. #1 using periodic table #2 using electron configuration. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. In this article today we are going. Bromine Valence Number.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Valence Number The first is to use the. There are two ways to find the number of valence electrons in bromine (br). #1 using periodic table #2 using electron configuration. Also, we will provide the pictures of the same. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Bromine has. Bromine Valence Number.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Valence Number Let’s break down each method in. Please read the full article below for more information. The first is to use the. Bromine has an electron configuration of #1s^2 2s^2 2p^6. What is the valency of bromine? We can write the valence electrons of bromine using two different methods: #1 using periodic table #2 using electron configuration. Also, we will provide. Bromine Valence Number.
From www.alamy.com
Bromine periodic Stock Vector Images Alamy Bromine Valence Number Bromine has an electron configuration of #1s^2 2s^2 2p^6. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). What is the valency of bromine? Please. Bromine Valence Number.
From mavink.com
Lewis Dot Diagram For Bromine Bromine Valence Number The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). There are two ways to find the number of valence electrons in bromine (br). What is the valency of bromine? Bromine has an electron configuration of #1s^2 2s^2 2p^6. In this article today we are going to tell. Bromine Valence Number.
From valenceelectrons.com
Silver(Ag) electron configuration and orbital diagram Bromine Valence Number The first is to use the. The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). Let’s break down each method in. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. We can write the. Bromine Valence Number.
From valenceelectrons.com
How to Find the Valence Electrons for Bromine (Br)? Bromine Valence Number Please read the full article below for more information. Valence electrons found in the s and p orbitals of the highest energy. Bromine has an electron configuration of #1s^2 2s^2 2p^6. Also, we will provide the pictures of the same. There are two ways to find the number of valence electrons in bromine (br). The ability of one atom of. Bromine Valence Number.
From www.youtube.com
Electron configuration, Orbital notation and Quantum Numbers for Bromine Valence Number The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). Let’s break down each method in. There are two ways to find the number of valence electrons in bromine (br). #1 using periodic table #2 using electron configuration. Also, we will provide the pictures of the same. What. Bromine Valence Number.
From wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry Bromine Valence Number The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. #1 using periodic table #2 using electron configuration. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond. Bromine Valence Number.
From periodictable.me
2000pxElectron_configuration_bromine.svg Dynamic Periodic Table of Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). Bromine has an electron configuration of #1s^2 2s^2 2p^6. Valence electrons found in the s and p orbitals of the highest energy. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Also, we. Bromine Valence Number.
From www.vecteezy.com
Bromine chemical element. Chemical symbol with atomic number and atomic Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). What is the valency of bromine? The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. The ability of one atom of an element. Bromine Valence Number.
From www.dreamstime.com
Periodic Table of Elements Bromine Stock Illustration Illustration Bromine Valence Number Please read the full article below for more information. In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. Bromine has an electron configuration of #1s^2 2s^2 2p^6. The total number of electrons present in the valence shell of an atom are called valence electrons, and there are. Bromine Valence Number.
From favpng.com
Electron Configuration Bromine Chemical Element Electron Shell Bohr Bromine Valence Number We can write the valence electrons of bromine using two different methods: There are two ways to find the number of valence electrons in bromine (br). Bromine has an electron configuration of #1s^2 2s^2 2p^6. What is the valency of bromine? 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will. Bromine Valence Number.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). The total number of electrons present in the valence shell of an atom are called valence electrons, and there are a total of seven electrons present in the valence shell of bromine. Let’s break down each method in. In this article today we are going to. Bromine Valence Number.
From proper-cooking.info
Bromine Valence Electrons Bromine Valence Number In this article today we are going to tell you about the electron configuration of bromine, its orbital diagram, and valence electron. The ability of one atom of an element to join another atom during the formation of a molecule is called valency (valence). The first is to use the. What is the valency of bromine? #1 using periodic table. Bromine Valence Number.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Valence Number There are two ways to find the number of valence electrons in bromine (br). Let’s break down each method in. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Valence electrons found in the s and p orbitals of the highest energy. The total number of electrons present. Bromine Valence Number.