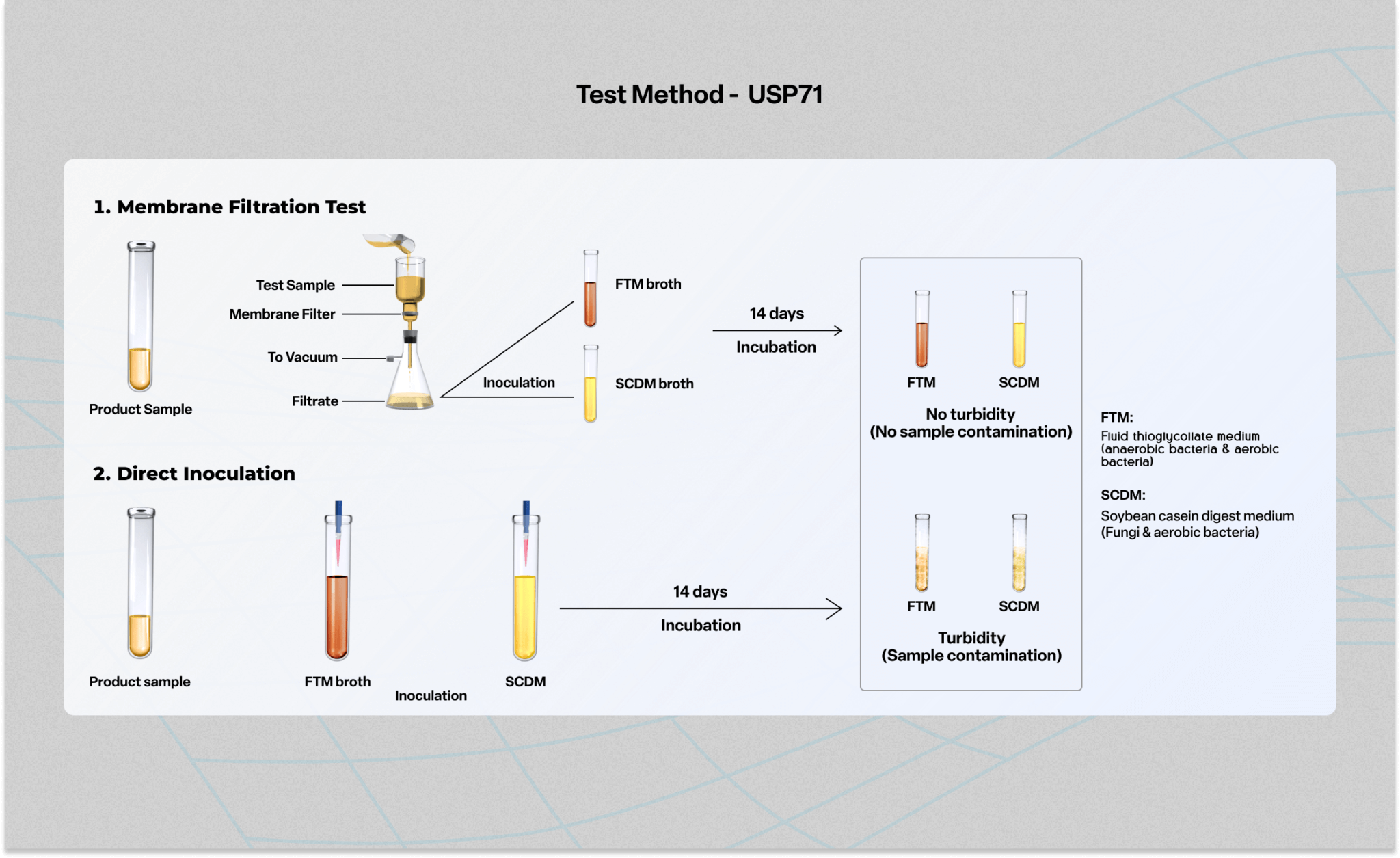
Membrane Filtration Method Usp . This approach relies upon the. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. Eur 2.6.1, and jp 4.06. The most probable number (mpn) method is generally the least. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane).
from microbe-investigations.com
The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). The most probable number (mpn) method is generally the least. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. Eur 2.6.1, and jp 4.06. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. This approach relies upon the.
USP 71 Sterility Testing Services Microbe Investigations
Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). The most probable number (mpn) method is generally the least. Eur 2.6.1, and jp 4.06. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. This approach relies upon the.
From ceodhagq.blob.core.windows.net
Membrane Filtration Method Sterility Testing at Shera Cooks blog Membrane Filtration Method Usp The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. Eur 2.6.1, and jp 4.06. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified. Membrane Filtration Method Usp.
From www.youtube.com
Membrane filtration to test for faecal contamination of water samples Membrane Filtration Method Usp An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. The membrane filtration sterility. Membrane Filtration Method Usp.
From klamgfpnl.blob.core.windows.net
Membrane Filter Types at Kim Greenwood blog Membrane Filtration Method Usp Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. This document describes the methods and procedures. Membrane Filtration Method Usp.
From mungfali.com
Membrane Filter Method Membrane Filtration Method Usp Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. This approach relies upon the. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such. Membrane Filtration Method Usp.
From oxymembrane.com
Membrane Filtration Method Oxy Membrane Membrane Filtration Method Usp Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). This document describes the methods and procedures for testing nonsterile products. Membrane Filtration Method Usp.
From www.pinterest.es
Membrane Filtration Method, Types, Advantages, Disadvantages Membrane Filtration Method Usp This approach relies upon the. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. The most probable number (mpn) method is generally the least. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,.. Membrane Filtration Method Usp.
From www.youtube.com
Membrane Filtration Method in Microbiology for Water Testing (in Membrane Filtration Method Usp An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. Eur 2.6.1, and jp 4.06. This approach relies upon the. The most probable number (mpn) method is generally the least. The membrane filtration method is the. Membrane Filtration Method Usp.
From www.mdpi.com
Membranes Free FullText UltraLowPressure Membrane Filtration for Membrane Filtration Method Usp Eur 2.6.1, and jp 4.06. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The membrane. Membrane Filtration Method Usp.
From in.pinterest.com
Membrane Filtration Method, Types, Advantages, Disadvantages, Applications. Membrane Filtration Method Usp The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). This approach relies upon the. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. Eur 2.6.1, and jp 4.06. This document describes the methods and procedures for testing nonsterile. Membrane Filtration Method Usp.
From www.researchgate.net
Illustration of membrane filtration Download Scientific Diagram Membrane Filtration Method Usp Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). The most probable number (mpn) method is generally the least. This document describes the methods and procedures for testing nonsterile products for the. Membrane Filtration Method Usp.
From www.slideserve.com
PPT Treat Waste Water by Membrane Filtration Process PowerPoint Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. The membrane. Membrane Filtration Method Usp.
From www.etch2o.com
Membrane Separation Water Treatment ETCH2O Membrane Filtration Method Usp Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. Eur 2.6.1, and jp 4.06. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. This approach relies upon the. The membrane filtration method is the method of choice where feasible (e.g., components are compatible. Membrane Filtration Method Usp.
From www.researchgate.net
(a) Schematic of filtration process and (b) vacuum filtration setup Membrane Filtration Method Usp The most probable number (mpn) method is generally the least. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the. Membrane Filtration Method Usp.
From www.youtube.com
Membrane Filtration Method UPLB Parasitology Research Laboratory Membrane Filtration Method Usp Eur 2.6.1, and jp 4.06. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. This approach. Membrane Filtration Method Usp.
From www.researchgate.net
Membrane filtration process Download Scientific Diagram Membrane Filtration Method Usp An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. This approach relies upon the. Eur 2.6.1, and jp 4.06. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with. Membrane Filtration Method Usp.
From watertp.tech
Membrane filtration • WaterTP Membrane Filtration Method Usp Eur 2.6.1, and jp 4.06. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with. Membrane Filtration Method Usp.
From www.researchgate.net
Schematic diagram of the membrane filtration system. Download Membrane Filtration Method Usp An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). The most probable number (mpn). Membrane Filtration Method Usp.
From www.pinterest.co.uk
Membrane Filtration Method, Types, Advantages, Disadvantages Membrane Filtration Method Usp The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such. Membrane Filtration Method Usp.
From microbe-investigations.com
USP 61 Testing for Microbial Enumeration Microbe Investigations Membrane Filtration Method Usp The most probable number (mpn) method is generally the least. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. This approach relies upon the. The membrane filtration method. Membrane Filtration Method Usp.
From www.pinterest.jp
Membrane Filtration Method, Types, Advantages, Disadvantages Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. This approach relies upon the. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. Eur 2.6.1, and jp 4.06. The most probable number (mpn). Membrane Filtration Method Usp.
From www.tpsearchtool.com
Membrane Filtration Method Types Advantages Disadvantages Applications Membrane Filtration Method Usp Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. Eur 2.6.1, and jp 4.06. This approach relies upon the. The most probable number (mpn) method is generally the least. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. The membrane filtration sterility test. Membrane Filtration Method Usp.
From klatvxwcl.blob.core.windows.net
Membrane Filtration Method For Water Testing at Anna blog Membrane Filtration Method Usp The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. This approach relies upon the. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. The membrane filtration method is the method of choice where. Membrane Filtration Method Usp.
From ami-journals.onlinelibrary.wiley.com
Membrane filtration immobilization technique—a simple and novel method Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. This approach. Membrane Filtration Method Usp.
From www.youtube.com
Coliform and Escherichia coli (E.Coli) Detection Membrane filtration Membrane Filtration Method Usp An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The most probable number (mpn) method is generally the least. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). Learn. Membrane Filtration Method Usp.
From www.cytivalifesciences.com
Microbiology membrane filter technique Cytiva Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The most probable number (mpn) method. Membrane Filtration Method Usp.
From netsolwater.com
What are the Types and degrees of membrane filtration Membrane Filtration Method Usp The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. This approach relies upon the. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane. Membrane Filtration Method Usp.
From microbe-investigations.com
USP 71 Sterility Testing Services Microbe Investigations Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The most probable number (mpn) method is generally the least. Eur 2.6.1, and jp 4.06. The membrane filtration sterility test is the. Membrane Filtration Method Usp.
From www.youtube.com
Sterility Testing Membrane Filtration YouTube Membrane Filtration Method Usp Learn how to perform usp 71 sterility testing for products labeled as sterile, using membrane filtration or direct inoculation. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). Eur 2.6.1, and jp 4.06. This approach relies upon the. The most probable number (mpn) method is generally the least. Learn how to. Membrane Filtration Method Usp.
From mavink.com
Membrane Filtration Apparatus Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). Learn how to perform. Membrane Filtration Method Usp.
From klatvxwcl.blob.core.windows.net
Membrane Filtration Method For Water Testing at Anna blog Membrane Filtration Method Usp This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Eur 2.6.1, and jp 4.06. This approach relies upon the. The membrane filtration method is the method of choice where feasible (e.g.,. Membrane Filtration Method Usp.
From synderfiltration.com
Synder Filtration Meets USP Class VI Certification Requirements in the Membrane Filtration Method Usp The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). Eur 2.6.1, and jp 4.06. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. This document describes the methods and. Membrane Filtration Method Usp.
From exomzvkxi.blob.core.windows.net
Membrane Filtration Method Can Be Used For Sterility Testing Of at Membrane Filtration Method Usp The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. The membrane filtration sterility test is the regulatory method of choice. Membrane Filtration Method Usp.
From www.researchgate.net
Schematic illustration of (a) the vacuum filtration method using AAO Membrane Filtration Method Usp The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). The most probable number (mpn) method is generally the least. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71, ph. Learn how to perform sterility tests for pharmacopeial articles using. Membrane Filtration Method Usp.
From www.etch2o.com
Membrane Separation In Industrial Effluent Treatment Etch2o Membrane Filtration Method Usp Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. This document describes the methods and procedures for testing nonsterile products for the presence or absence of specified microorganisms, such as staphylococcus aureus,. The membrane filtration sterility test is the regulatory method of choice for filterable pharmaceutical products, as cited in the usp 71,. Membrane Filtration Method Usp.
From ceyoervs.blob.core.windows.net
Membrane Filtration Usp at Kayla Sager blog Membrane Filtration Method Usp Learn how to perform sterility tests for pharmacopeial articles using membrane filtration or direct inoculation methods. An approach that is often used, especially in sterility testing, is neutralization by membrane filtration. The membrane filtration method is the method of choice where feasible (e.g., components are compatible with the membrane). This approach relies upon the. The membrane filtration sterility test is. Membrane Filtration Method Usp.