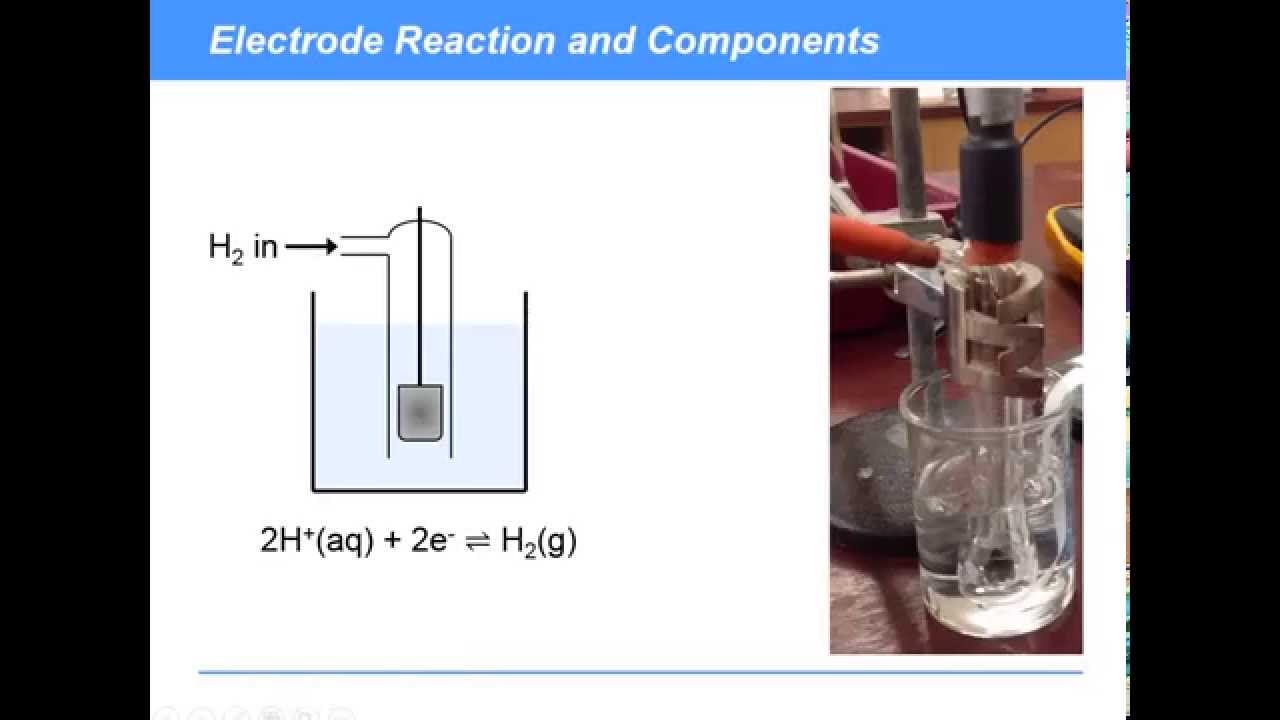
Standard Hydrogen Electrode Electrochemical Cell . She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells.
from www.youtube.com
This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Hydrogen gas in equilibrium with h.
KAC32.4 Electrochemistry Standard Hydrogen Electrode YouTube
Standard Hydrogen Electrode Electrochemical Cell She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. Hydrogen gas in equilibrium with h. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k.
From cartoondealer.com
A Standard Hydrogen Electrode (SHE) Is An Electrode That Scientists Use Standard Hydrogen Electrode Electrochemical Cell Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. She is a. Standard Hydrogen Electrode Electrochemical Cell.
From www.askiitians.com
Electrode Potential Study Material for IIT JEE askIITians Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Although the standard hydrogen electrode is one of the most fundamental concepts of. Standard Hydrogen Electrode Electrochemical Cell.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Electrochemical Cell She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is constructed, and. Standard Hydrogen Electrode Electrochemical Cell.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential. Standard Hydrogen Electrode Electrochemical Cell.
From www.mdpi.com
Batteries Free FullText Electrochemical Testing of Carbon Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its. Standard Hydrogen Electrode Electrochemical Cell.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Electrochemical Cell Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0. Standard Hydrogen Electrode Electrochemical Cell.
From app.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Learn what a standard hydrogen electrode (she) is, how it is constructed, and. Standard Hydrogen Electrode Electrochemical Cell.
From mavink.com
Electrochemical Cell Diagram Standard Hydrogen Electrode Electrochemical Cell This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at. Standard Hydrogen Electrode Electrochemical Cell.
From www.palmsens.com
BASI MF1051 Standard Electrochemical Cell PalmSens Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k.. Standard Hydrogen Electrode Electrochemical Cell.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Hydrogen gas in equilibrium with h. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Learn what a standard hydrogen electrode (she) is, how it is constructed, and. Standard Hydrogen Electrode Electrochemical Cell.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity. Standard Hydrogen Electrode Electrochemical Cell.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Standard Hydrogen Electrode Electrochemical Cell Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential. Standard Hydrogen Electrode Electrochemical Cell.
From www.slideserve.com
PPT Redox Geochemistry PowerPoint Presentation, free download ID Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of She is a reference electrode for comparing the reducing power of any element and has. Standard Hydrogen Electrode Electrochemical Cell.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes. Standard Hydrogen Electrode Electrochemical Cell.
From mmerevise.co.uk
Electrochemical Cells MME Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its. Standard Hydrogen Electrode Electrochemical Cell.
From www.youtube.com
KAC32.4 Electrochemistry Standard Hydrogen Electrode YouTube Standard Hydrogen Electrode Electrochemical Cell This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. Although the standard hydrogen electrode. Standard Hydrogen Electrode Electrochemical Cell.
From www.pinterest.com
Electrochemical cell Electrochemistry, Chemistry classroom Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Hydrogen gas in equilibrium with h. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential. Standard Hydrogen Electrode Electrochemical Cell.
From rohanfersmorrison.blogspot.com
Identify the Conditions for a Standard Electrochemical Cell. Standard Hydrogen Electrode Electrochemical Cell Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v. Standard Hydrogen Electrode Electrochemical Cell.
From chemistry.stackexchange.com
electrochemistry Where do negative ions migrate from the salt bridge Standard Hydrogen Electrode Electrochemical Cell This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn how to. Standard Hydrogen Electrode Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential. Standard Hydrogen Electrode Electrochemical Cell.
From www.pinterest.com
Standard Hydrogen Electrode in 2020 Electron configuration, Ideal Standard Hydrogen Electrode Electrochemical Cell Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential. Standard Hydrogen Electrode Electrochemical Cell.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Standard Hydrogen Electrode Electrochemical Cell Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. This article. Standard Hydrogen Electrode Electrochemical Cell.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Standard Hydrogen Electrode Electrochemical Cell Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its. Standard Hydrogen Electrode Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Electrochemical Cell Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v. Standard Hydrogen Electrode Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Hydrogen gas in. Standard Hydrogen Electrode Electrochemical Cell.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Electrochemical Cell Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Learn what a. Standard Hydrogen Electrode Electrochemical Cell.
From wisc.pb.unizin.org
D40.3 Standard HalfCell Potentials Chemistry 109 Fall 2021 Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its. Standard Hydrogen Electrode Electrochemical Cell.
From loegddcbq.blob.core.windows.net
Standard Hydrogen Electrode In English at Janice Wagner blog Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Hydrogen gas in equilibrium with h. This article reviews the theory, design, operation, and applications of standard and reversible. Standard Hydrogen Electrode Electrochemical Cell.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Standard Hydrogen Electrode Electrochemical Cell She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells.. Standard Hydrogen Electrode Electrochemical Cell.
From klaefpten.blob.core.windows.net
Electrochemical And Electrolytic Cell Comparison at Luther Estes blog Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at. Standard Hydrogen Electrode Electrochemical Cell.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Standard Hydrogen Electrode Electrochemical Cell Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. Hydrogen gas in equilibrium with h. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Although the standard hydrogen electrode is one of the most fundamental concepts. Standard Hydrogen Electrode Electrochemical Cell.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of Hydrogen gas in. Standard Hydrogen Electrode Electrochemical Cell.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Standard Hydrogen Electrode Electrochemical Cell Although the standard hydrogen electrode is one of the most fundamental concepts of physical chemistry and its potential is defined as 0.000 v at any temperature as long as the activity of the hydrated proton and the fugacity of This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. She is a. Standard Hydrogen Electrode Electrochemical Cell.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Hydrogen Electrode Electrochemical Cell Learn how to use the standard hydrogen electrode to measure the potentials of other electrodes in electrochemical cells. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. She is a reference electrode for comparing the reducing power of any element and has a standard electrode potential of 0 at 298k. Although. Standard Hydrogen Electrode Electrochemical Cell.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Electrochemical Cell Hydrogen gas in equilibrium with h. Learn what a standard hydrogen electrode (she) is, how it is constructed, and what its half cell reaction is. This article reviews the theory, design, operation, and applications of standard and reversible hydrogen electrodes in electrocatalysis and. She is a reference electrode for comparing the reducing power of any element and has a standard. Standard Hydrogen Electrode Electrochemical Cell.