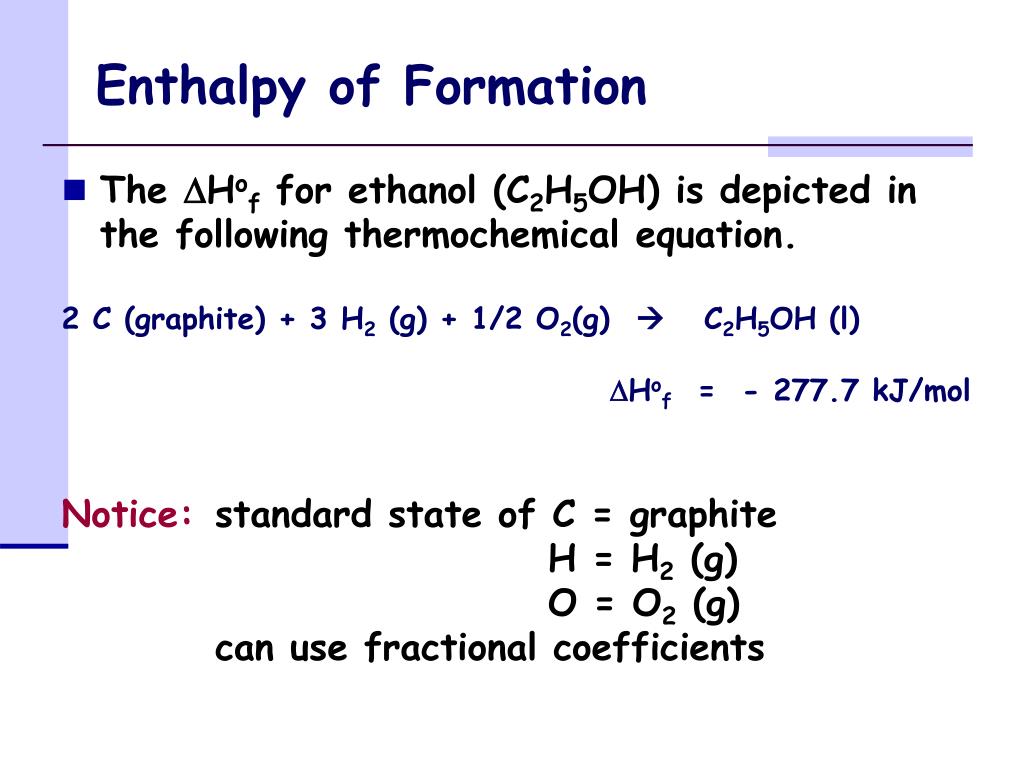
Aluminum Hydroxide Enthalpy Of Formation . Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. However, allotropes of an element not in the standard state typically do have enthalpy values. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. The formation of any chemical can be as a reaction from the corresponding elements: Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of an element in its standard state is zero. \[ \text{elements} \rightarrow \text{compound} \nonumber\]
from www.slideserve.com
Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The formation of any chemical can be as a reaction from the corresponding elements: However, allotropes of an element not in the standard state typically do have enthalpy values. The enthalpy of an element in its standard state is zero. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products.
PPT Enthalpy of Formation PowerPoint Presentation, free download ID
Aluminum Hydroxide Enthalpy Of Formation \[ \text{elements} \rightarrow \text{compound} \nonumber\] Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. \[ \text{elements} \rightarrow \text{compound} \nonumber\] The formation of any chemical can be as a reaction from the corresponding elements: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. However, allotropes of an element not in the standard state typically do have enthalpy values. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. The enthalpy of an element in its standard state is zero.
From schematicfixpulpits.z21.web.core.windows.net
Energy Cycle Diagram Aluminum Hydroxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,.. Aluminum Hydroxide Enthalpy Of Formation.
From aluminumhydroxidesaidan.blogspot.com
Aluminum Hydroxide Standard Formation Reaction Of Solid Aluminum Hydroxide Aluminum Hydroxide Enthalpy Of Formation Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. The enthalpy. Aluminum Hydroxide Enthalpy Of Formation.
From www.chemistrystudent.com
Hess' Law and Hess Cycles (ALevel) ChemistryStudent Aluminum Hydroxide Enthalpy Of Formation However, allotropes of an element not in the standard state typically do have enthalpy values. Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Decomposition of pure aluminium hydroxide. Aluminum Hydroxide Enthalpy Of Formation.
From www.semanticscholar.org
Lithium aluminum‐layered double hydroxide chlorides ( LDH ) Formation Aluminum Hydroxide Enthalpy Of Formation \[ \text{elements} \rightarrow \text{compound} \nonumber\] The formation of any chemical can be as a reaction from the corresponding elements: Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. The enthalpy of an element in its standard state is zero. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Aluminum Hydroxide Enthalpy Of Formation.
From www.youtube.com
Calculate the enthalpy of formation of anhydrous aluminium chloride, Al Aluminum Hydroxide Enthalpy Of Formation Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. However, allotropes of an element not in the standard state typically do have enthalpy values. Enthalpy of formation (\(δh_f\)) is. Aluminum Hydroxide Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Aluminum Hydroxide Enthalpy Of Formation Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. However, allotropes of an element not in the standard state typically do have enthalpy values. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. However, allotropes of an element not in the standard state typically do have enthalpy values. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT Formation Reactions PowerPoint Presentation, free download ID Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. However, allotropes of an element not in the standard state typically do have enthalpy values. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. 193 rows. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Aluminum Hydroxide Enthalpy Of Formation Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. \[ \text{elements} \rightarrow \text{compound}. Aluminum Hydroxide Enthalpy Of Formation.
From slideplayer.com
Enthalpy of formation 2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(s) ppt download Aluminum Hydroxide Enthalpy Of Formation Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component. Aluminum Hydroxide Enthalpy Of Formation.
From www.semanticscholar.org
Lithium aluminum‐layered double hydroxide chlorides ( LDH ) Formation Aluminum Hydroxide Enthalpy Of Formation Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. However, allotropes of an element not in the standard state typically do have enthalpy values.. Aluminum Hydroxide Enthalpy Of Formation.
From narodnatribuna.info
Heat Of Formation And Enthalpy Data For Slag Compounds Aluminum Hydroxide Enthalpy Of Formation The formation of any chemical can be as a reaction from the corresponding elements: However, allotropes of an element not in the standard state typically do have enthalpy values. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The enthalpy of an element in its standard. Aluminum Hydroxide Enthalpy Of Formation.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Aluminum Hydroxide Enthalpy Of Formation Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. The enthalpy of an element in its standard state is zero. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon. Aluminum Hydroxide Enthalpy Of Formation.
From www.researchgate.net
Enthalpy as a function of surface area for aluminum oxides and Aluminum Hydroxide Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. The formation of any chemical can be as a reaction from the corresponding elements: Calculate the change in enthalpy for a reaction using the heat of formation values. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Calculate the change in enthalpy for a. Aluminum Hydroxide Enthalpy Of Formation.
From mungfali.com
Enthalpies Of Formation Chart Aluminum Hydroxide Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. \[ \text{elements} \rightarrow \text{compound} \nonumber\] The formation of any chemical can be as a reaction from the corresponding elements: 193 rows in chemistry and thermodynamics, the standard enthalpy. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID3528115 Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. However, allotropes of an element not in the standard state typically do have enthalpy values. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Decomposition of pure aluminium hydroxide has been studied in various papers and its. Aluminum Hydroxide Enthalpy Of Formation.
From www.researchgate.net
Standard Gibbs free energy of formation, standard enthalpy of formation Aluminum Hydroxide Enthalpy Of Formation The formation of any chemical can be as a reaction from the corresponding elements: Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy. Aluminum Hydroxide Enthalpy Of Formation.
From www.youtube.com
Balance Al + H2O = Al(OH)3 + H2 (Aluminum and Water) YouTube Aluminum Hydroxide Enthalpy Of Formation \[ \text{elements} \rightarrow \text{compound} \nonumber\] Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component. Aluminum Hydroxide Enthalpy Of Formation.
From classhirsch.z21.web.core.windows.net
Standard Enthalpy Of Formation Chart Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. Decomposition of pure aluminium hydroxide has been. Aluminum Hydroxide Enthalpy Of Formation.
From slideplayer.com
Enthalpy of formation 2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(s) ppt download Aluminum Hydroxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. \[ \text{elements} \rightarrow \text{compound} \nonumber\] However, allotropes of an element not in the standard state typically do have enthalpy values. The enthalpy of an element in its standard state is zero. 193 rows in chemistry and thermodynamics, the standard. Aluminum Hydroxide Enthalpy Of Formation.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Aluminum Hydroxide Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. However, allotropes of an element not in. Aluminum Hydroxide Enthalpy Of Formation.
From saylordotorg.github.io
Enthalpy Aluminum Hydroxide Enthalpy Of Formation \[ \text{elements} \rightarrow \text{compound} \nonumber\] Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. Definition and explanation of the terms standard. Aluminum Hydroxide Enthalpy Of Formation.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Aluminum Hydroxide Enthalpy Of Formation Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. \[ \text{elements} \rightarrow \text{compound} \nonumber\] However, allotropes. Aluminum Hydroxide Enthalpy Of Formation.
From www.youtube.com
Enthalpies of Reactions Using Average Bond Enthalpies Chemistry Aluminum Hydroxide Enthalpy Of Formation \[ \text{elements} \rightarrow \text{compound} \nonumber\] Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. However, allotropes. Aluminum Hydroxide Enthalpy Of Formation.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Aluminum Hydroxide Enthalpy Of Formation \[ \text{elements} \rightarrow \text{compound} \nonumber\] The formation of any chemical can be as a reaction from the corresponding elements: Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change. Aluminum Hydroxide Enthalpy Of Formation.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Aluminum Hydroxide Enthalpy Of Formation Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of an element in its standard state is zero. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. The formation of any. Aluminum Hydroxide Enthalpy Of Formation.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Aluminum Hydroxide Enthalpy Of Formation Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of an element in its standard state is zero. However, allotropes of an element not in the standard state typically do. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Aluminum Hydroxide Enthalpy Of Formation However, allotropes of an element not in the standard state typically do have enthalpy values. The enthalpy of an element in its standard state is zero. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. 193 rows in chemistry and thermodynamics, the standard enthalpy of. Aluminum Hydroxide Enthalpy Of Formation.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1549724 Aluminum Hydroxide Enthalpy Of Formation Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. Decomposition of. Aluminum Hydroxide Enthalpy Of Formation.
From mungfali.com
Enthalpies Of Formation Table Aluminum Hydroxide Enthalpy Of Formation Enthalpy of formation (\(δh_f\)) is the enthalpy change for the formation of 1 mol of a compound from its component elements, such as the formation of carbon dioxide from carbon and oxygen. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy. Aluminum Hydroxide Enthalpy Of Formation.
From hollyburton.z19.web.core.windows.net
Enthalpy Of Formation Chart Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Calculate the change in enthalpy for a reaction using the heat of formation values of the reactants and products. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. However, allotropes of an element not in the standard. Aluminum Hydroxide Enthalpy Of Formation.
From www.researchgate.net
Example aluminium hydroxide and hydroxyaluminosilicate structures Aluminum Hydroxide Enthalpy Of Formation Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. The enthalpy of an element in its standard state is zero. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of. Aluminum Hydroxide Enthalpy Of Formation.
From chem.libretexts.org
5.7 Enthalpies of Formation Chemistry LibreTexts Aluminum Hydroxide Enthalpy Of Formation 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The enthalpy of an element in its standard state is zero. Calculate the change in enthalpy. Aluminum Hydroxide Enthalpy Of Formation.
From byjus.com
Calculate the enthalpy of formation of anhydrous Al2Cl6 from the Aluminum Hydroxide Enthalpy Of Formation The enthalpy of an element in its standard state is zero. Decomposition of pure aluminium hydroxide has been studied in various papers and its decomposition reaction mechanism,. \[ \text{elements} \rightarrow \text{compound} \nonumber\] Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Enthalpy of formation (\(δh_f\)) is the enthalpy. Aluminum Hydroxide Enthalpy Of Formation.