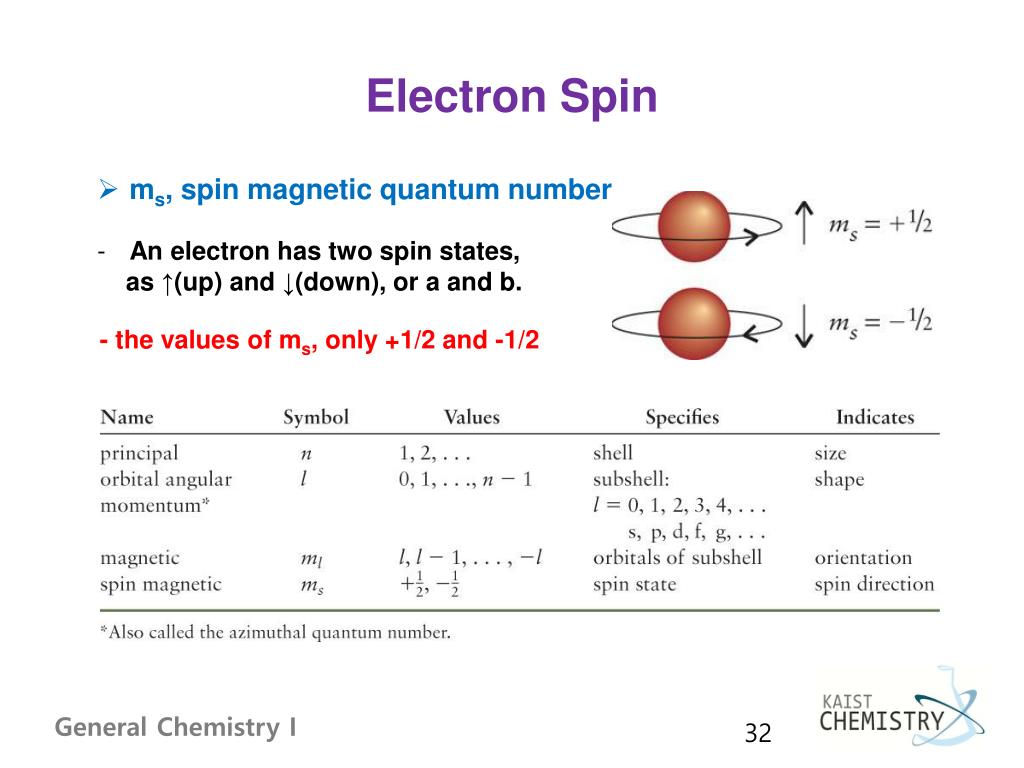
Spin Property Of Electron . The electron spin is one of the three inherent properties of the electrons; The electron spin is described as the electron. An electron spin s = 1/2 is an intrinsic property of electrons. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin up and down allows two electrons for each set of spatial quantum numbers. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. The others are the mass and charge of the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,.
from www.slideserve.com
Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. An electron spin s = 1/2 is an intrinsic property of electrons. The electron spin is described as the electron. The electron spin is one of the three inherent properties of the electrons; The others are the mass and charge of the electron. Spin up and down allows two electrons for each set of spatial quantum numbers. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules.
PPT QUANTUM MECHANICS AND ATOMIC STRUCTURE PowerPoint Presentation
Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spin s = 1/2 is an intrinsic property of electrons. The others are the mass and charge of the electron. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is described as the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. The electron spin is one of the three inherent properties of the electrons;
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra Spin Property Of Electron An electron spin s = 1/2 is an intrinsic property of electrons. Spin up and down allows two electrons for each set of spatial quantum numbers. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. In a. Spin Property Of Electron.
From www.scienceabc.com
How Electron Spin Contributes To The Zeeman Effect And Predicting Star Spin Property Of Electron An electron spin s = 1/2 is an intrinsic property of electrons. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin is a fundamental characteristic of all particles, not just electrons, and is. Spin Property Of Electron.
From www.slideserve.com
PPT QUANTUM MECHANICS AND ATOMIC STRUCTURE PowerPoint Presentation Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The others are the mass and charge of the electron. The electron spin is one of the three inherent properties of the electrons; The electron spin is described as the electron. Spin up and down allows two electrons for each set of spatial. Spin Property Of Electron.
From www.slideserve.com
PPT Many electron atoms and the Periodic Table PowerPoint Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The electron spin is described as the electron. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. The others are. Spin Property Of Electron.
From www.slideserve.com
PPT Chapter 6 Electronic Structure of Atoms PowerPoint Presentation Spin Property Of Electron Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. The electron spin is one of the three inherent properties of the electrons; Spin up and down allows two electrons for each set of spatial quantum numbers. In a broader sense, spin is an essential property influencing the ordering of electrons and. Spin Property Of Electron.
From fullstackquantumcomputation.tech
Quantum Dots and their Application as Electron Spin Qubits FullStack Spin Property Of Electron Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. The electron spin is described as the electron. The others. Spin Property Of Electron.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. An electron spin s = 1/2 is an intrinsic property of electrons. The electron spin is described as the electron. Each type of. Spin Property Of Electron.
From www.toppr.com
What is Electron Spin Theory of Electron Spin, Directions, Formula, Spin Property Of Electron The electron spin is described as the electron. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms. Spin Property Of Electron.
From www.slideserve.com
PPT Quantum Phenomena II Matter Matters PowerPoint Presentation Spin Property Of Electron The others are the mass and charge of the electron. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. The electron spin is described as the electron. The electron spin is one of the three inherent properties of the electrons; Electron spin or spin quantum number is the fourth quantum number. Spin Property Of Electron.
From discover.hubpages.com
Particle Physics Why Do Electrons Have Spin of Half? HubPages Spin Property Of Electron The others are the mass and charge of the electron. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. An electron spin s = 1/2 is an intrinsic property of electrons. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin is a. Spin Property Of Electron.
From slideplayer.com
Electronic Structure of the Atom ppt download Spin Property Of Electron Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The electron spin is described as the electron. The others are. Spin Property Of Electron.
From slideplayer.com
from Electric Current ppt download Spin Property Of Electron Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spin s = 1/2 is an intrinsic property of. Spin Property Of Electron.
From chem.libretexts.org
6.7 One More Electron Property Electron Spin Chemistry LibreTexts Spin Property Of Electron In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Spin up and down allows two electrons for each set of spatial quantum numbers. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. An electron spin s = 1/2 is an. Spin Property Of Electron.
From www.slideserve.com
PPT Chapter Seven Atomic Structure PowerPoint Presentation, free Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The electron spin is described as the electron. The others are the mass and charge of the electron. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes,. Spin Property Of Electron.
From www.youtube.com
Bob's model electron spin YouTube Spin Property Of Electron The others are the mass and charge of the electron. An electron spin s = 1/2 is an intrinsic property of electrons. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin. Spin Property Of Electron.
From www.slideserve.com
PPT Chapter 8 Periodic Properties of the Elements PowerPoint Spin Property Of Electron Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The others are the mass and charge of the electron. Spin up and down allows two electrons for each set of spatial quantum numbers. Spin. Spin Property Of Electron.
From www.slideserve.com
PPT PowerPoint Presentation, free download ID2172594 Spin Property Of Electron The others are the mass and charge of the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spin s = 1/2 is an intrinsic property of electrons. The electron spin is one of the three inherent properties of the electrons; Each type of particle has a characteristic spin,. Spin Property Of Electron.
From www.youtube.com
Introduction to Electron Spin Part 1 YouTube Spin Property Of Electron The electron spin is one of the three inherent properties of the electrons; In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. The electron spin is described as the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin up. Spin Property Of Electron.
From www.physics.rutgers.edu
Electron Spin Holds the Key Spin Property Of Electron The others are the mass and charge of the electron. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Spin up and down allows two electrons for each set of spatial. Spin Property Of Electron.
From www.slideserve.com
PPT Atomic Theory PowerPoint Presentation, free download ID6568018 Spin Property Of Electron In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in atoms and molecules,. Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is described as the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Electron. Spin Property Of Electron.
From www.thoughtco.com
What Is Electron Spin in Chemistry? Spin Property Of Electron An electron spin s = 1/2 is an intrinsic property of electrons. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin up and down allows two electrons for each set of spatial quantum numbers. In a broader sense, spin is an essential property influencing the ordering of electrons and nuclei in. Spin Property Of Electron.
From www.slideserve.com
PPT Chapter 4 “Electron Configurations” PowerPoint Presentation, free Spin Property Of Electron The electron spin is one of the three inherent properties of the electrons; Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin up and down allows two electrons for each set of spatial quantum numbers. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the. Spin Property Of Electron.
From slideplayer.com
How can you express the arrangement of electrons in atoms through Spin Property Of Electron The electron spin is one of the three inherent properties of the electrons; The others are the mass and charge of the electron. Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Spin up and down allows two electrons for each set of spatial quantum numbers. Spin is a fundamental characteristic. Spin Property Of Electron.
From physics.aps.org
Physics Nuclear Spins Tango to the Tune of an Electron Spin Property Of Electron An electron spin s = 1/2 is an intrinsic property of electrons. The others are the mass and charge of the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is described as the. Spin Property Of Electron.
From www.secretsofuniverse.in
What Does An Electron's Spin Actually Represent And How Was It Spin Property Of Electron Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. An electron spin s = 1/2 is an intrinsic property of electrons. The electron spin is described as the electron. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about. Spin Property Of Electron.
From jpsht.jps.jp
Spin Transport in a TwoDimensional Tilted Dirac Electron System JPS Spin Property Of Electron The electron spin is described as the electron. Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is one of the three inherent properties of the electrons; Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. Spin is a fundamental characteristic of all. Spin Property Of Electron.
From www.slideserve.com
PPT Electron Configuration and Atomic Properties PowerPoint Spin Property Of Electron Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The electron spin is described as the electron. Spin up and. Spin Property Of Electron.
From www.expii.com
Electron Spin — Definition & Overview Expii Spin Property Of Electron The electron spin is described as the electron. The others are the mass and charge of the electron. Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. In a broader sense, spin is an essential property influencing. Spin Property Of Electron.
From slideplayer.com
Quantum Theory & the Atom ppt download Spin Property Of Electron Spin is a fundamental characteristic of all particles, not just electrons, and is analogous to the intrinsic spin of extended bodies about their own axes, such as the daily rotation of earth. The electron spin is described as the electron. An electron spin s = 1/2 is an intrinsic property of electrons. Spin up and down allows two electrons for. Spin Property Of Electron.
From www.youtube.com
Atomic Structure 07 Electron Spin and Pairing YouTube Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spin s = 1/2 is an intrinsic property of electrons. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The electron spin is one of the three inherent properties of the electrons; The. Spin Property Of Electron.
From inf.news
The first discovery of electron spin iNEWS Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. The others are the mass and charge of the electron. An electron spin s = 1/2 is an intrinsic property of electrons. The electron spin is described as the electron. In a broader sense, spin is an essential property influencing the ordering of. Spin Property Of Electron.
From studylib.net
Understanding Electron Spin Spin Property Of Electron The electron spin is one of the three inherent properties of the electrons; Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. In a broader sense, spin is an essential property influencing the ordering of. Spin Property Of Electron.
From www.slideserve.com
PPT Electron Configurations and Quantum Chemistry PowerPoint Spin Property Of Electron Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is one of the three inherent properties of the electrons; Each type of particle has a characteristic spin, which is quantized and expressed in units of the reduced. An electron spin s = 1/2 is an intrinsic property of electrons. The others are. Spin Property Of Electron.
From www.slideserve.com
PPT Ch 6 PowerPoint Presentation, free download ID2948406 Spin Property Of Electron Electron spin or spin quantum number is the fourth quantum number for electrons in atoms and molecules. An electron spin s = 1/2 is an intrinsic property of electrons. Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is one of the three inherent properties of the electrons; In a broader sense,. Spin Property Of Electron.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra Spin Property Of Electron The electron spin is one of the three inherent properties of the electrons; Spin up and down allows two electrons for each set of spatial quantum numbers. The electron spin is described as the electron. The others are the mass and charge of the electron. Electron spin or spin quantum number is the fourth quantum number for electrons in atoms. Spin Property Of Electron.