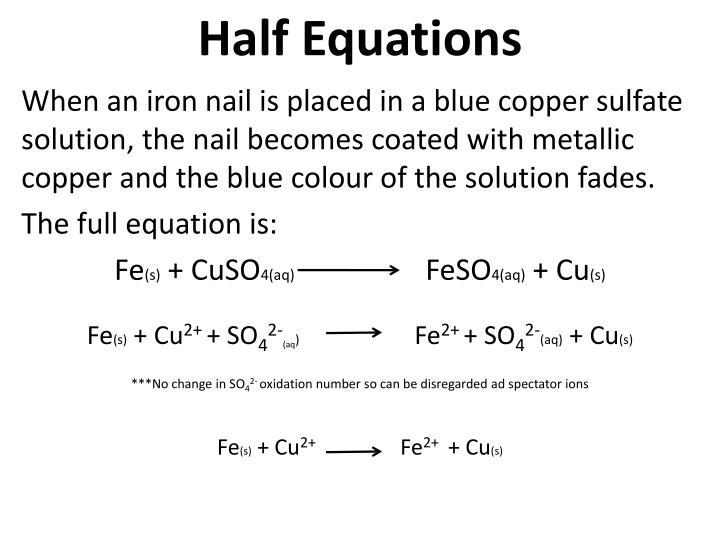
Zinc And Copper Sulfate Redox Reaction . Reaction of zinc and copper(ii) sulfate. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. What happens when you add zinc to a solution of copper sulfate? To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Zn + cuso4 → znso4 + cu. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately. Identifying the half reactions to see what got oxidized and reduced. Consider the reaction of zinc metal with copper(ii) sulfate: Copper (cu) is deposited in its metallic form from the solution. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s).
from www.slideserve.com
Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. Reaction of zinc and copper(ii) sulfate. Consider the reaction of zinc metal with copper(ii) sulfate: What happens when you add zinc to a solution of copper sulfate? To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Zn + cuso4 → znso4 + cu. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately.
PPT Redox Reactions PowerPoint Presentation ID3872488
Zinc And Copper Sulfate Redox Reaction Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Zn + cuso4 → znso4 + cu. Copper (cu) is deposited in its metallic form from the solution. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Consider the reaction of zinc metal with copper(ii) sulfate: When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Reaction of zinc and copper(ii) sulfate. What happens when you add zinc to a solution of copper sulfate? Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. Identifying the half reactions to see what got oxidized and reduced.
From www.numerade.com
SOLVED The reaction between zinc and copper sulphate is illustrated Zinc And Copper Sulfate Redox Reaction What happens when you add zinc to a solution of copper sulfate? To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Reaction of zinc and copper(ii) sulfate. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Copper (cu) is. Zinc And Copper Sulfate Redox Reaction.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Sulfate Redox Reaction Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Zn + cuso4 → znso4 + cu. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Zn +. Zinc And Copper Sulfate Redox Reaction.
From klazoofqu.blob.core.windows.net
Zinc Hydroxide And Sulfuric Acid at Mamie Hunter blog Zinc And Copper Sulfate Redox Reaction What happens when you add zinc to a solution of copper sulfate? Zn + cuso4 → znso4 + cu. Consider the reaction of zinc metal with copper(ii) sulfate: Copper (cu) is deposited in its metallic form from the solution. Reaction of zinc and copper(ii) sulfate. When a strip of zinc metal is placed into a blue solution of copper (ii). Zinc And Copper Sulfate Redox Reaction.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Sulfate Redox Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Consider the reaction of zinc metal with copper(ii) sulfate: Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one. Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
Zinc and Copper Sulphate reaction (Displacement Reaction) Zn and CuSO4 Zinc And Copper Sulfate Redox Reaction Consider the reaction of zinc metal with copper(ii) sulfate: Zn + cuso4 → znso4 + cu. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Identifying the half reactions to see what got oxidized and reduced. When a strip of. Zinc And Copper Sulfate Redox Reaction.
From www.chegg.com
Solved The reaction between zinc and copper sulphate is Zinc And Copper Sulfate Redox Reaction Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Copper (cu) is deposited in its metallic form from the solution. When a strip of zinc metal is placed into a. Zinc And Copper Sulfate Redox Reaction.
From www.slideserve.com
PPT Galvanic (= voltaic) Cells PowerPoint Presentation, free download Zinc And Copper Sulfate Redox Reaction Copper (cu) is deposited in its metallic form from the solution. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Consider the reaction of zinc metal with copper(ii) sulfate: What happens when you add zinc to a solution of copper sulfate? Cu 2+ + 2e − → cu copper is reduced from +2 to. Zinc And Copper Sulfate Redox Reaction.
From www.pastpapersinside.com
Redox Reaction Past Papers Inside Zinc And Copper Sulfate Redox Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Copper (cu) is. Zinc And Copper Sulfate Redox Reaction.
From klaexmnbf.blob.core.windows.net
Copper Iodide Redox Reaction at Robert Marx blog Zinc And Copper Sulfate Redox Reaction Zn + cuso4 → znso4 + cu. What happens when you add zinc to a solution of copper sulfate? Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Copper. Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
The reaction between Zinc and Copper Sulfate YouTube Zinc And Copper Sulfate Redox Reaction Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Consider the reaction of zinc metal with copper(ii) sulfate: What happens. Zinc And Copper Sulfate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental Zinc And Copper Sulfate Redox Reaction Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Identifying the half reactions to see what got oxidized and reduced. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. When a strip of zinc metal is placed. Zinc And Copper Sulfate Redox Reaction.
From fineartamerica.com
Zinc Reacting With Copper Sulfate Photograph by GIPhotoStock Zinc And Copper Sulfate Redox Reaction In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Reaction of zinc and copper(ii) sulfate. When a strip of zinc metal is. Zinc And Copper Sulfate Redox Reaction.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation ID3872488 Zinc And Copper Sulfate Redox Reaction To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Zn + cuso4 → znso4 + cu. Copper (cu) is deposited in its metallic form from the solution. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq). Zinc And Copper Sulfate Redox Reaction.
From www.slideshare.net
Replacement reactions Zinc And Copper Sulfate Redox Reaction Consider the reaction of zinc metal with copper(ii) sulfate: Identifying the half reactions to see what got oxidized and reduced. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid. Zinc And Copper Sulfate Redox Reaction.
From www.teachoo.com
MCQ (Sample Paper) In reaction of iron with copper sulphate solution Zinc And Copper Sulfate Redox Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. What happens when you add zinc to a solution of copper sulfate? To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in. Zinc And Copper Sulfate Redox Reaction.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Sulfate Redox Reaction Reaction of zinc and copper(ii) sulfate. To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Zn + cuso4 → znso4 + cu. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. In the reaction between zinc and copper. Zinc And Copper Sulfate Redox Reaction.
From www.coursehero.com
[Solved] link for the experiment https//youtu.be/ILJhI43wVA Part Zinc And Copper Sulfate Redox Reaction Identifying the half reactions to see what got oxidized and reduced. To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. What happens when you add zinc to a solution of copper sulfate? When a strip of zinc metal is placed into a blue solution. Zinc And Copper Sulfate Redox Reaction.
From www.thesciencehive.co.uk
Redox and Electrode Potentials* — the science sauce Zinc And Copper Sulfate Redox Reaction Copper (cu) is deposited in its metallic form from the solution. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately. Zn + cuso4 → znso4 + cu. What happens when you add zinc to a solution of copper sulfate? When a strip of zinc metal is placed into. Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc And Copper Sulfate Redox Reaction Reaction of zinc and copper(ii) sulfate. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. What happens when you add zinc to a solution of copper. Zinc And Copper Sulfate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental Zinc And Copper Sulfate Redox Reaction In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Copper (cu) is deposited in its metallic form from the solution. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Consider the reaction of zinc metal with copper(ii) sulfate: When a strip. Zinc And Copper Sulfate Redox Reaction.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Zinc And Copper Sulfate Redox Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. Copper (cu) is deposited. Zinc And Copper Sulfate Redox Reaction.
From www.dreamstime.com
Single Displacement Reaction Iron Nail in Copper Sulfate Solution Zinc And Copper Sulfate Redox Reaction Zn + cuso4 → znso4 + cu. Copper (cu) is deposited in its metallic form from the solution. What happens when you add zinc to a solution of copper sulfate? Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. When a strip of zinc metal is placed into a blue solution of copper (ii). Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc And Copper Sulfate Redox Reaction To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Consider the reaction of zinc metal with copper(ii) sulfate: Copper (cu) is deposited in its metallic form from the solution. When. Zinc And Copper Sulfate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction iron copper sulfate Fundamental Zinc And Copper Sulfate Redox Reaction Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4. Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
single displacement reaction of Copper from Copper Sulphate Solution by Zinc And Copper Sulfate Redox Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken. Copper (cu) is deposited in its metallic form from the solution. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn]. Zinc And Copper Sulfate Redox Reaction.
From www.sciencephoto.com
Nickel Reacting With Copper Sulfate Stock Image C028/0162 Science Zinc And Copper Sulfate Redox Reaction Cu 2+ + 2e − → cu copper is reduced from +2 to 0. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Zn + cuso4 = cu + znso4 is a. Zinc And Copper Sulfate Redox Reaction.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II Zinc And Copper Sulfate Redox Reaction When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately. Reaction of zinc and copper(ii) sulfate. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a. Zinc And Copper Sulfate Redox Reaction.
From www.toppr.com
ii) When zinc granule is dipped into copper sulphate solution, copper Zinc And Copper Sulfate Redox Reaction Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zn + cuso4 = cu + znso4 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and one mole of aqueous cupric sulfate. Reaction of zinc and copper(ii) sulfate. Consider the reaction of zinc metal with copper(ii) sulfate: What happens when you. Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
Redox reaction from dissolving zinc in copper sulfate Chemistry Zinc And Copper Sulfate Redox Reaction Copper (cu) is deposited in its metallic form from the solution. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. When a strip of zinc metal is placed into a blue solution of. Zinc And Copper Sulfate Redox Reaction.
From www.youtube.com
Zinc and copper sulfate react Investigating Chemical Reactions Zinc And Copper Sulfate Redox Reaction Reaction of zinc and copper(ii) sulfate. Copper (cu) is deposited in its metallic form from the solution. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s). Zinc And Copper Sulfate Redox Reaction.
From fphoto.photoshelter.com
science chemistry redox reaction zinc Fundamental Photographs The Zinc And Copper Sulfate Redox Reaction Reaction of zinc and copper(ii) sulfate. To show that the chemical reaction between zinc and copper sulfate dissolved in water is a redox reaction in terms of the electronic concept, we. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately begins as the zinc strip begins to darken.. Zinc And Copper Sulfate Redox Reaction.
From byjus.com
14.During indirect redox reaction why there is a release of zinc ions Zinc And Copper Sulfate Redox Reaction Cu 2+ + 2e − → cu copper is reduced from +2 to 0. Zn → zn 2+ + 2e − zinc is oxidized from 0 to +2. Zn + cuso4 → znso4 + cu. Identifying the half reactions to see what got oxidized and reduced. When a strip of zinc metal is placed into a blue solution of copper. Zinc And Copper Sulfate Redox Reaction.
From www.slideshare.net
Redox= quiz part 1 with answers Zinc And Copper Sulfate Redox Reaction In the reaction between zinc and copper (ii) sulfate, the ionic equation is zn(s) + cuso4 (aq) → znso4 (aq) + cu(s). Zn + cuso4 → znso4 + cu. Reaction of zinc and copper(ii) sulfate. Identifying the half reactions to see what got oxidized and reduced. When a strip of zinc metal is placed into a blue solution of copper. Zinc And Copper Sulfate Redox Reaction.
From www.shalom-education.com
Displacement Reactions GCSE Chemistry Revision Zinc And Copper Sulfate Redox Reaction Consider the reaction of zinc metal with copper(ii) sulfate: Zinc metal reacts with copper sulphate cuso 4 solution to form zinc sulphate znso 4. Zn + cuso4 → znso4 + cu. Identifying the half reactions to see what got oxidized and reduced. Reaction of zinc and copper(ii) sulfate. To show that the chemical reaction between zinc and copper sulfate dissolved. Zinc And Copper Sulfate Redox Reaction.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Sulfate Redox Reaction Copper (cu) is deposited in its metallic form from the solution. Cu 2+ + 2e − → cu copper is reduced from +2 to 0. When a strip of zinc metal is placed into a blue solution of copper (ii) sulfate (figure below), a reaction immediately. Zn + cuso4 → znso4 + cu. When a strip of zinc metal is. Zinc And Copper Sulfate Redox Reaction.