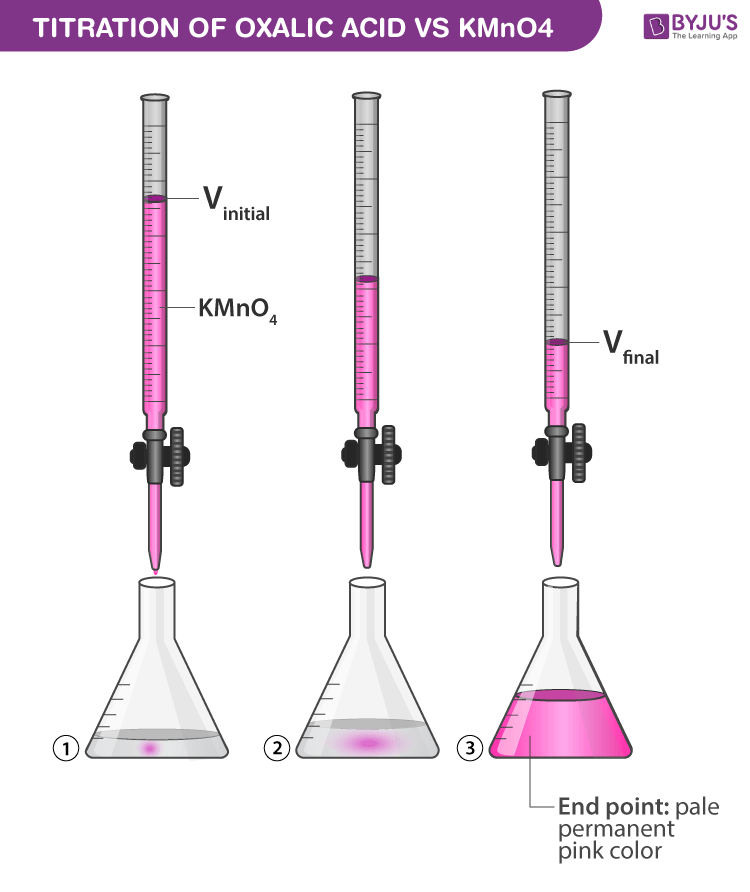
Indicator For Oxalic Acid Titration . In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. In close proximity to the endpoint, the action of the. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration.
from byjus.com
The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent.
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12
Indicator For Oxalic Acid Titration In close proximity to the endpoint, the action of the. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the endpoint, the action of the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent.
From www.youtube.com
Titration of Oxalic acid vs KMnO4B.Sc 1st sem (NEP)Dr.Chhavi Purwar Indicator For Oxalic Acid Titration In close proximity to the endpoint, the action of the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint ,. Indicator For Oxalic Acid Titration.
From www.studypool.com
SOLUTION chemistry Practical layout to find strength of Indicator For Oxalic Acid Titration In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close. Indicator For Oxalic Acid Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Oxalic Acid Titration In close proximity to the endpoint, the action of the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases. Indicator For Oxalic Acid Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the. Indicator For Oxalic Acid Titration.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the endpoint, the action of the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The point at which a color change is observed is the endpoint ,. Indicator For Oxalic Acid Titration.
From askfilo.com
What is the suitable indicator for titration of NaOH and oxalic acid.. Indicator For Oxalic Acid Titration In close proximity to the endpoint, the action of the. The point at which a color change is observed is the endpoint , which is close to the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c. Indicator For Oxalic Acid Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the. Indicator For Oxalic Acid Titration.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the endpoint, the action of the. The point at which a color change is observed is the endpoint ,. Indicator For Oxalic Acid Titration.
From www.youtube.com
Redox Titration II KMnO4 vs Oxalic acid II Self indicator YouTube Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The point. Indicator For Oxalic Acid Titration.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The point at which a color change is observed is the endpoint , which is close to. Indicator For Oxalic Acid Titration.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The point. Indicator For Oxalic Acid Titration.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Indicator For Oxalic Acid Titration The point at which a color change is observed is the endpoint , which is close to the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno. Indicator For Oxalic Acid Titration.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. In close. Indicator For Oxalic Acid Titration.
From www.numerade.com
SOLVED (2 Pts) You decide to titrate the solution of NaOH using oxalic Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the. Indicator For Oxalic Acid Titration.
From www.studypool.com
SOLUTION chemistry Practical layout to find strength of Indicator For Oxalic Acid Titration In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point. Indicator For Oxalic Acid Titration.
From www.numerade.com
SOLVED Experiment A Titration using an indicator A1. Preparation of Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves. Indicator For Oxalic Acid Titration.
From www.toppr.com
What is the indicator used for the titration between oxalic acid and Indicator For Oxalic Acid Titration The point at which a color change is observed is the endpoint , which is close to the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno. Indicator For Oxalic Acid Titration.
From www.chegg.com
Solved 8. Oxalic acid concentration may be determined in a Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the endpoint, the action of the. They are typically weak acids or bases whose changes in. Indicator For Oxalic Acid Titration.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the endpoint, the action of the. The point at which a color change is observed is the endpoint ,. Indicator For Oxalic Acid Titration.
From www.youtube.com
Titration of NaOH with oxalic acid using phenolphthalein indicator Indicator For Oxalic Acid Titration The point at which a color change is observed is the endpoint , which is close to the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the. Indicator For Oxalic Acid Titration.
From www.doubtnut.com
Which of following indicators is used in the titration of oxalic acid Indicator For Oxalic Acid Titration In close proximity to the endpoint, the action of the. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint ,. Indicator For Oxalic Acid Titration.
From www.toppr.com
What is the indicator used for the titration between oxalic acid and Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint ,. Indicator For Oxalic Acid Titration.
From mungfali.com
Acid Base Titration Indicator Indicator For Oxalic Acid Titration The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color. Indicator For Oxalic Acid Titration.
From www.numerade.com
SOLVED The graph below shows the titration of oxalic acid, C2H2O4 Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The point at which a color change is observed is the endpoint , which is close to the. They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the. Indicator For Oxalic Acid Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The point at which a color change is observed is the endpoint , which. Indicator For Oxalic Acid Titration.
From askfilo.com
In this titration of KMnO4 vs oxalic acid, what is the indicator used? Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while. Indicator For Oxalic Acid Titration.
From www.scribd.com
BCB 103L Expt 6 Titration of Sodium Hydroxide With Oxalic Acid Using Indicator For Oxalic Acid Titration The point at which a color change is observed is the endpoint , which is close to the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The titration of potassium permanganate (kmno. Indicator For Oxalic Acid Titration.
From www.youtube.com
Titration Of NaOH with Oxalic Acid ( Class XI, Practical1) YouTube Indicator For Oxalic Acid Titration In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which is close to the. The titration of potassium permanganate (kmno. Indicator For Oxalic Acid Titration.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration Indicator For Oxalic Acid Titration The point at which a color change is observed is the endpoint , which is close to the. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c. Indicator For Oxalic Acid Titration.
From flatworldknowledge.lardbucket.org
Quantitative Analysis Using Titrations Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The point at which a color change is observed is the endpoint , which is close to. Indicator For Oxalic Acid Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. The point at which a color change is observed is the endpoint , which is close to the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing. Indicator For Oxalic Acid Titration.
From www.chegg.com
Solved Tap on the equivalence point Titration Curve (oxalic Indicator For Oxalic Acid Titration In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. They are typically weak acids or bases whose changes in color correspond to deprotonation or. The point at which a color change is observed is the endpoint , which. Indicator For Oxalic Acid Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Indicator For Oxalic Acid Titration They are typically weak acids or bases whose changes in color correspond to deprotonation or. In this titration process, the oxalic acid acts as the reducing agent while potassium permanganate (kmno4) serves as the oxidizing agent. The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close. Indicator For Oxalic Acid Titration.
From www.numerade.com
SOLVED Explain how the indicator works in the oxalate titration Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. They are typically weak acids or bases whose changes in color correspond to deprotonation or. In close proximity to the endpoint, the action of the. In this titration process, the oxalic acid acts as the reducing agent while. Indicator For Oxalic Acid Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Indicator For Oxalic Acid Titration The titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. In close proximity to the endpoint, the action of the. The point at which a color change is observed is the endpoint , which is close to the. They are typically weak acids or bases whose changes in. Indicator For Oxalic Acid Titration.