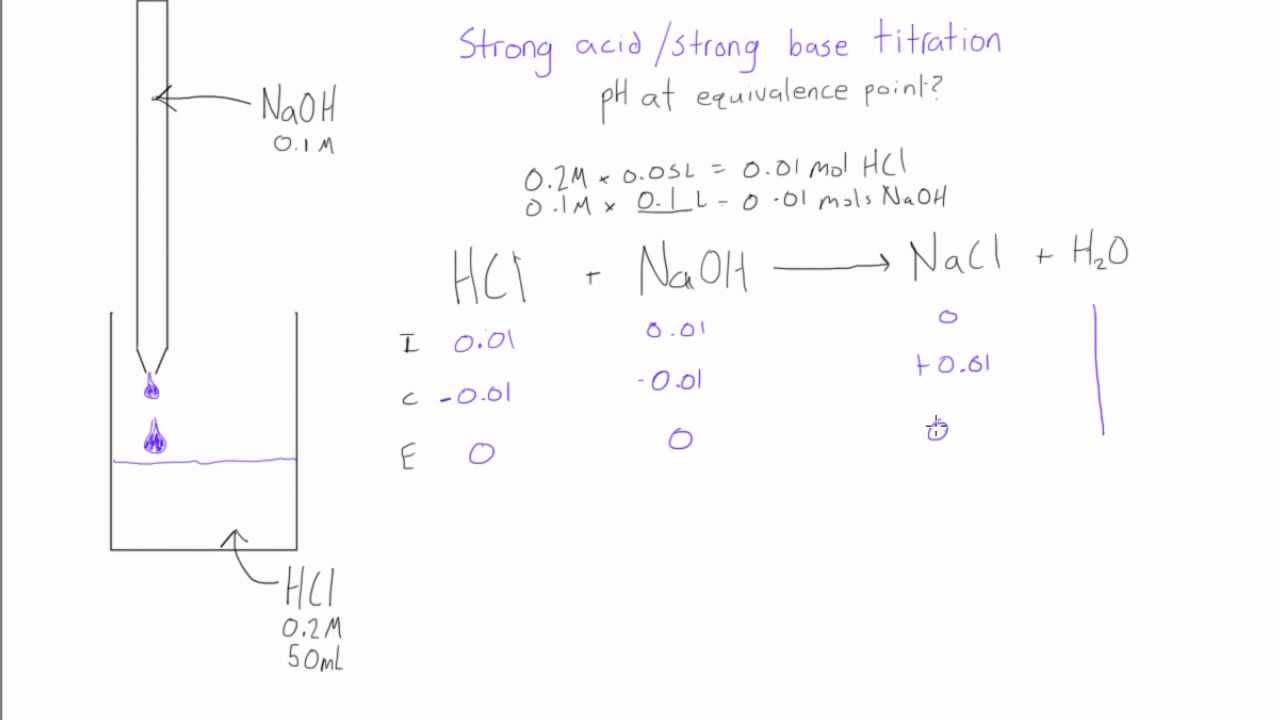
Equivalence Point Acid Titration . The equivalence point of a titration. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. in chemistry, an equivalence point is a term that is used while performing titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. Sorting out some confusing terms.
from www.youtube.com
to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. Sorting out some confusing terms. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. in chemistry, an equivalence point is a term that is used while performing titration. The equivalence point of a titration.
Strong acid / strong base titration pH at equivalence point YouTube
Equivalence Point Acid Titration The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. The equivalence point of a titration. in chemistry, an equivalence point is a term that is used while performing titration. Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Equivalence Point Acid Titration Sorting out some confusing terms. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. The equivalence point of a titration. in chemistry, an equivalence point is a term that is used while performing titration. the equivalence point or stoichiometric point is the point in a chemical reaction. Equivalence Point Acid Titration.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction. Equivalence Point Acid Titration.
From www.youtube.com
What is Equivalence Point (acidbase titrations)? YouTube Equivalence Point Acid Titration The equivalence point of a titration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point, or stoichiometric point, of a chemical. Equivalence Point Acid Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Equivalence Point Acid Titration in chemistry, an equivalence point is a term that is used while performing titration. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. the. Equivalence Point Acid Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Equivalence Point Acid Titration Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. The equivalence point of a titration. in chemistry, an equivalence point is a term that is used while performing titration. the equivalence point or stoichiometric point is the point in a chemical reaction. Equivalence Point Acid Titration.
From www.numerade.com
SOLVED ' The graph shows the titration curves for two monoprotic acids, (a) Which Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. Sorting out some confusing terms. in chemistry, an equivalence point is a term that is used while performing titration. The equivalence point of a titration. In a titration, it is where the moles of titrant equal the moles of. Equivalence Point Acid Titration.
From oneclass.com
CHE 2B Study Guide Spring 2019, Final Titration, Equivalence Point, Acid Dissociation Constant Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. Sorting out some confusing terms. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. to evaluate the relationship between a titration’s equivalence point and its end point, we need to. Equivalence Point Acid Titration.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Equivalence Point Acid Titration Sorting out some confusing terms. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. The equivalence point of a titration. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. in chemistry, an equivalence point is a term that is. Equivalence Point Acid Titration.
From saylordotorg.github.io
AcidBase Titrations Equivalence Point Acid Titration The equivalence point of a titration. Sorting out some confusing terms. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. In a titration,. Equivalence Point Acid Titration.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Equivalence Point Acid Titration in chemistry, an equivalence point is a term that is used while performing titration. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. In a titration, it is. Equivalence Point Acid Titration.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Equivalence Point Acid Titration Sorting out some confusing terms. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. to evaluate the relationship between a titration’s equivalence point and. Equivalence Point Acid Titration.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic acid, H2C2O4, and sodium Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a titration. in chemistry, an equivalence point is a. Equivalence Point Acid Titration.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid with a strong base, if the Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. in chemistry, an equivalence point is a term that is used while performing titration. Sorting. Equivalence Point Acid Titration.
From www.numerade.com
Based on the titration curve shown below, what's the pKa of the acid being titrated? Titration Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. The equivalence point of a titration. Sorting out some confusing terms. in chemistry, an equivalence point is a term that is used while performing titration. to evaluate the relationship between a titration’s equivalence point and its end point,. Equivalence Point Acid Titration.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID5369229 Equivalence Point Acid Titration The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. Sorting out some confusing terms. in chemistry, an equivalence point is a term that is used while performing titration. the equivalence point or stoichiometric point is the point in a chemical reaction. Equivalence Point Acid Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators of Acid Base Titration Equivalence Point Acid Titration to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. Sorting out some confusing terms. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the equivalence point or stoichiometric point is the point in a chemical reaction when. Equivalence Point Acid Titration.
From www.researchgate.net
Titration curve of acetic acid at 30 °C (Ve = equivalence volume) Download Scientific Diagram Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. Sorting out some confusing terms. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. in chemistry, an equivalence point is a term that is used while performing titration. The equivalence. Equivalence Point Acid Titration.
From www.vrogue.co
How To Calculate Pka From The Half Equivalence Point vrogue.co Equivalence Point Acid Titration in chemistry, an equivalence point is a term that is used while performing titration. The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only. Equivalence Point Acid Titration.
From billyfersarias.blogspot.com
Calculate the Ph at the Equivalence Point Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the. Equivalence Point Acid Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. Sorting out some confusing terms. in chemistry, an equivalence point is a term that is used while performing titration. the. Equivalence Point Acid Titration.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Equivalence Point Acid Titration Sorting out some confusing terms. The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. in chemistry, an equivalence point is a term. Equivalence Point Acid Titration.
From goodttorials.blogspot.com
How To Find Equivalence Point From Titration Data Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the equivalence point or stoichiometric point is the point in a chemical reaction when there. Equivalence Point Acid Titration.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax General Chemistry Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. Sorting out some confusing terms. The equivalence point of a titration. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. in chemistry, an equivalence point is a term that is. Equivalence Point Acid Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Equivalence Point Acid Titration to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. in chemistry, an equivalence point is a term that is used while performing titration. the equivalence point or. Equivalence Point Acid Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base. Equivalence Point Acid Titration.
From dxomvqiez.blob.core.windows.net
Equivalence Point For Titration Of Strong Acid at Ora Peach blog Equivalence Point Acid Titration the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. in chemistry, an equivalence point is a term that is used while performing. Equivalence Point Acid Titration.
From mungfali.com
Equivalence Points On Titration Graph Equivalence Point Acid Titration In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base. Equivalence Point Acid Titration.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a titration. in chemistry, an equivalence point is a. Equivalence Point Acid Titration.
From www.vrogue.co
Point In Acid Base Titration The Equivalence Point So vrogue.co Equivalence Point Acid Titration to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. in chemistry, an equivalence point is a term that is used while performing titration. Sorting out some confusing terms. The equivalence point of a titration. In a titration, it is where the moles of titrant equal the moles of. Equivalence Point Acid Titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Equivalence Point Acid Titration in chemistry, an equivalence point is a term that is used while performing titration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only. Equivalence Point Acid Titration.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Equivalence Point Acid Titration The equivalence point of a titration. to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In a titration, it is where the moles. Equivalence Point Acid Titration.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Chemistry Power Point WITH Equivalence Point Acid Titration The equivalence point of a titration. the equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. Sorting out some confusing terms. in chemistry,. Equivalence Point Acid Titration.
From dxomvqiez.blob.core.windows.net
Equivalence Point For Titration Of Strong Acid at Ora Peach blog Equivalence Point Acid Titration to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. The equivalence point of a titration. the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. the equivalence point or stoichiometric point is the point in a chemical reaction. Equivalence Point Acid Titration.
From www.youtube.com
Titration Weak base/strong acid Before the equivalence point YouTube Equivalence Point Acid Titration the equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of. The equivalence point of a titration. in chemistry, an equivalence point is a term that is used while performing titration. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. Sorting. Equivalence Point Acid Titration.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators of Acid Base Titration Equivalence Point Acid Titration to evaluate the relationship between a titration’s equivalence point and its end point, we need to construct only a. In a titration, it is where the moles of titrant equal the moles of solution of unknown concentration. The equivalence point of a titration. Sorting out some confusing terms. in chemistry, an equivalence point is a term that is. Equivalence Point Acid Titration.