Bomb Calorimeter Experiment Manual . There are two basic types of calorimeters: Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. One of the most important types of. Four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. Heats of combustion are most common, in which the combustible material is. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bucket or container for holding the bomb in a. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. A bomb calorimeter will be calibrated using a sample of known benzoic.
from www.chegg.com
Heats of combustion are most common, in which the combustible material is. A bomb or vessel in which the combustible charges can be burned. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Four essential parts are required in any bomb calorimeter: Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. There are two basic types of calorimeters: One of the most important types of. A bucket or container for holding the bomb in a. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bomb calorimeter will be calibrated using a sample of known benzoic.
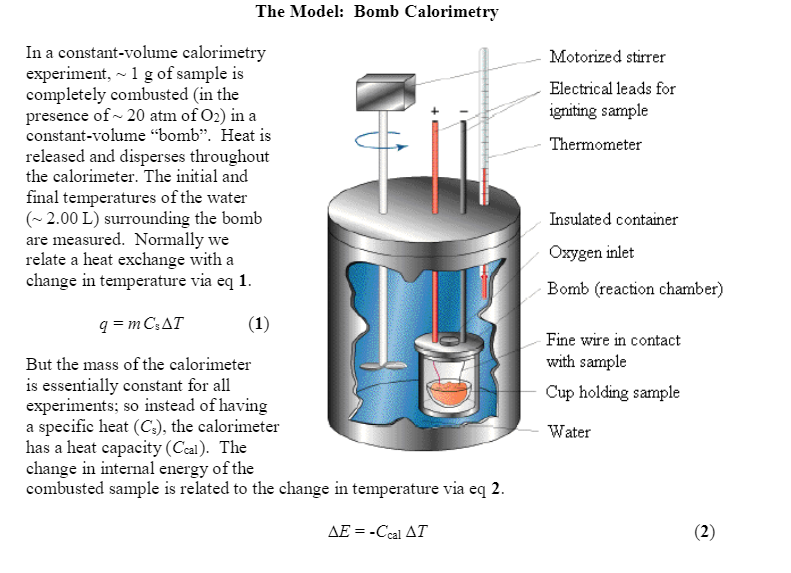
Solved The Model Bomb Calorimetry Motorized stirrer
Bomb Calorimeter Experiment Manual One of the most important types of. There are two basic types of calorimeters: One of the most important types of. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bomb calorimeter will be calibrated using a sample of known benzoic. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. Heats of combustion are most common, in which the combustible material is. A bucket or container for holding the bomb in a.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Experiment Manual There are two basic types of calorimeters: Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h. Bomb Calorimeter Experiment Manual.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimeter Experiment Manual Heats of combustion are most common, in which the combustible material is. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. There are two basic types of calorimeters: A bomb or vessel in which the combustible charges can be burned. One of. Bomb Calorimeter Experiment Manual.
From socratic.org
When 0.602 g of biphenyl (C12H10) undergoes combustion in a bomb Bomb Calorimeter Experiment Manual One of the most important types of. A bomb or vessel in which the combustible charges can be burned. Heats of combustion are most common, in which the combustible material is. A bomb calorimeter will be calibrated using a sample of known benzoic. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. Four. Bomb Calorimeter Experiment Manual.
From gamma.app
Bomb Calorimeter A Comprehensive Guide Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bomb calorimeter will be calibrated using a sample of known benzoic. There are two basic types of calorimeters: Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. In this experiment, you will use a sample of benzoic acid to. Bomb Calorimeter Experiment Manual.
From ar.inspiredpencil.com
Bomb Calorimeter Setup Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. There are two basic types of calorimeters: A bomb or vessel in which the combustible charges can be burned. Heats of combustion are most common, in which the combustible material is. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this.. Bomb Calorimeter Experiment Manual.
From byjus.com
What is bomb calorimeter? Bomb Calorimeter Experiment Manual Four essential parts are required in any bomb calorimeter: One of the most important types of. There are two basic types of calorimeters: A bucket or container for holding the bomb in a. A bomb calorimeter will be calibrated using a sample of known benzoic. In this experiment, you will use a sample of benzoic acid to determine the ccal. Bomb Calorimeter Experiment Manual.
From nanbei-newchina.en.made-in-china.com
Xry1A Manual Cheap Coal Calorific Value Testing Equipment Oxygen Bomb Bomb Calorimeter Experiment Manual In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bucket or container for holding the bomb in a. A bomb calorimeter will be calibrated using a sample of known benzoic. One of the most important types of. Most tabulated h values. Bomb Calorimeter Experiment Manual.
From courses.lumenlearning.com
Calorimetry Chemistry Bomb Calorimeter Experiment Manual In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bucket or container for holding the bomb in a. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: One of. Bomb Calorimeter Experiment Manual.
From www.slideshare.net
Bomb calorimeter experiment Bomb Calorimeter Experiment Manual Heats of combustion are most common, in which the combustible material is. A bucket or container for holding the bomb in a. One of the most important types of. There are two basic types of calorimeters: A bomb calorimeter will be calibrated using a sample of known benzoic. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter. Bomb Calorimeter Experiment Manual.
From www.chegg.com
Solved The Model Bomb Calorimetry Motorized stirrer Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bucket or container for holding the bomb in a. A bomb calorimeter will be calibrated using a sample of known benzoic. One of the most important types of. There are two basic types of calorimeters: In this experiment, you will use a sample of benzoic acid. Bomb Calorimeter Experiment Manual.
From www.chegg.com
Solved A bomb calorimeter, or a constant volume calorimeter, Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. A bomb or vessel in which the combustible charges can be burned. Four essential parts are required in any bomb calorimeter: In this experiment, you will use a sample of benzoic. Bomb Calorimeter Experiment Manual.
From animalia-life.club
Simple Bomb Calorimeter Bomb Calorimeter Experiment Manual Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. A bomb or vessel in which the combustible charges can be burned. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. Heats of combustion are most common, in which the combustible material is. Four essential parts are required in any. Bomb Calorimeter Experiment Manual.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Experiment Manual A bomb calorimeter will be calibrated using a sample of known benzoic. One of the most important types of. Heats of combustion are most common, in which the combustible material is. There are two basic types of calorimeters: A bucket or container for holding the bomb in a. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance. Bomb Calorimeter Experiment Manual.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Bomb Calorimeter Experiment Manual One of the most important types of. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. There are two basic types of calorimeters: A bucket or. Bomb Calorimeter Experiment Manual.
From www.slideserve.com
PPT ME 4611 Experiment 5 Bomb Calorimeter Experiment PowerPoint Bomb Calorimeter Experiment Manual In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. One of the most important types of. A bucket or container for holding the bomb in a. Heats of combustion are most common, in which the combustible material is. A bomb or vessel. Bomb Calorimeter Experiment Manual.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Experiment Manual Four essential parts are required in any bomb calorimeter: A bomb calorimeter will be calibrated using a sample of known benzoic. One of the most important types of. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Most tabulated h values of. Bomb Calorimeter Experiment Manual.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Bomb Calorimeter Experiment Manual In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Heats of combustion are most common, in which the combustible material is. Four essential parts are required in any bomb calorimeter: A bucket or container for holding the bomb in a. Most tabulated. Bomb Calorimeter Experiment Manual.
From www.studocu.com
CHEM 201 Lab Manual 2023 v9 B EXPT. B HEAT OF COMBUSTION BY BOMB Bomb Calorimeter Experiment Manual In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bucket or container for holding the bomb in a. Heats of combustion are most common, in which the combustible material is. Most tabulated h values of highly exothermic reactions come from “bomb”. Bomb Calorimeter Experiment Manual.
From www.studocu.com
Exp1 Bomb+Calorimetry A B Experiment 1 Determination of enthalpy of Bomb Calorimeter Experiment Manual There are two basic types of calorimeters: Four essential parts are required in any bomb calorimeter: A bomb calorimeter will be calibrated using a sample of known benzoic. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. One of the most important types of. In this experiment, you will use a sample of. Bomb Calorimeter Experiment Manual.
From www.scribd.com
Experiment 1 Bomb Calorimetry Calorimetry Heat Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. Heats of combustion are most common, in which the combustible material is. A bucket or container for holding the bomb in a. Four essential parts are required in any bomb calorimeter: In this experiment, you will use a sample of benzoic acid to determine the ccal of. Bomb Calorimeter Experiment Manual.
From www.scribd.com
Manual For Experiment With Bomb Calorimeter PDF Combustion Enthalpy Bomb Calorimeter Experiment Manual A bomb calorimeter will be calibrated using a sample of known benzoic. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bomb or vessel in which the combustible charges can be burned. One of the most important types of.. Bomb Calorimeter Experiment Manual.
From www.scribd.com
MANUAL FOR BOMB CALORIMETER.doc British Thermal Unit Combustion Bomb Calorimeter Experiment Manual Four essential parts are required in any bomb calorimeter: In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bucket or container for holding the bomb in a. A. Bomb Calorimeter Experiment Manual.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Experiment Manual A bucket or container for holding the bomb in a. There are two basic types of calorimeters: A bomb calorimeter will be calibrated using a sample of known benzoic. One of the most important types of. Heats of combustion are most common, in which the combustible material is. In this experiment, you will use a sample of benzoic acid to. Bomb Calorimeter Experiment Manual.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Bomb Calorimeter Experiment Manual Four essential parts are required in any bomb calorimeter: In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. A bomb or vessel in which the combustible. Bomb Calorimeter Experiment Manual.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. Four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare. Bomb Calorimeter Experiment Manual.
From www.youtube.com
Thermophysical characterization Bomb calorimeter (Standard Operating Bomb Calorimeter Experiment Manual In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. Four essential parts are required in any bomb calorimeter: Heats of combustion are most common, in which the combustible material is. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance. Bomb Calorimeter Experiment Manual.
From studylib.net
STANDARD OPERATING PROCEDURE AND SAFETY GUIDE FOR BOMB CALORIMETER Bomb Calorimeter Experiment Manual Four essential parts are required in any bomb calorimeter: There are two basic types of calorimeters: A bomb calorimeter will be calibrated using a sample of known benzoic. Heats of combustion are most common, in which the combustible material is. A bucket or container for holding the bomb in a. Most tabulated h values of highly exothermic reactions come from. Bomb Calorimeter Experiment Manual.
From www.slideserve.com
PPT ME 4611 Experiment 5 Bomb Calorimeter Experiment PowerPoint Bomb Calorimeter Experiment Manual One of the most important types of. Four essential parts are required in any bomb calorimeter: A bomb or vessel in which the combustible charges can be burned. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bomb calorimeter will be. Bomb Calorimeter Experiment Manual.
From www.slideshare.net
2 pa32 bomb calorimeter procedure Bomb Calorimeter Experiment Manual Four essential parts are required in any bomb calorimeter: Heats of combustion are most common, in which the combustible material is. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bucket or container for holding the bomb in a. Most tabulated. Bomb Calorimeter Experiment Manual.
From studylib.net
Bomb Calorimeter Lab Sheet Bomb Calorimeter Experiment Manual There are two basic types of calorimeters: Heats of combustion are most common, in which the combustible material is. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. Four essential parts are required in any bomb calorimeter: In this experiment, you will use a sample of benzoic acid to determine the ccal of. Bomb Calorimeter Experiment Manual.
From studylib.net
ME 4611 Experiment 5 Bomb Calorimeter Experiment Bomb Calorimeter Experiment Manual A bucket or container for holding the bomb in a. One of the most important types of. There are two basic types of calorimeters: A bomb or vessel in which the combustible charges can be burned. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. Four essential parts are required in any bomb calorimeter: In this. Bomb Calorimeter Experiment Manual.
From studylib.net
Bomb Calorimeter Bomb Calorimeter Experiment Manual Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bomb or vessel in which the combustible charges can be burned. One of the most important types of. Heats of combustion are most common, in which the combustible material is. A bomb calorimeter will be calibrated using a sample of known benzoic. In this experiment, you. Bomb Calorimeter Experiment Manual.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Experiment Manual A bomb or vessel in which the combustible charges can be burned. Most tabulated h values of highly exothermic reactions come from “bomb” calorimeter experiments. A bomb calorimeter will be calibrated using a sample of known benzoic. Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. There are two basic types of calorimeters:. Bomb Calorimeter Experiment Manual.
From fewcontent.ashokhall.com
Divine Info About How To Build A Bomb Calorimeter Fewcontent Bomb Calorimeter Experiment Manual Bomb calorimeter the enthalpy of combustion of naphthalene and an unknown substance is determined in this. In this experiment, you will use a sample of benzoic acid to determine the ccal of your bomb calorimeter and a value of ∆h to compare with. A bomb or vessel in which the combustible charges can be burned. Most tabulated h values of. Bomb Calorimeter Experiment Manual.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Experiment Manual A bomb or vessel in which the combustible charges can be burned. A bomb calorimeter will be calibrated using a sample of known benzoic. A bucket or container for holding the bomb in a. Heats of combustion are most common, in which the combustible material is. There are two basic types of calorimeters: Bomb calorimeter the enthalpy of combustion of. Bomb Calorimeter Experiment Manual.