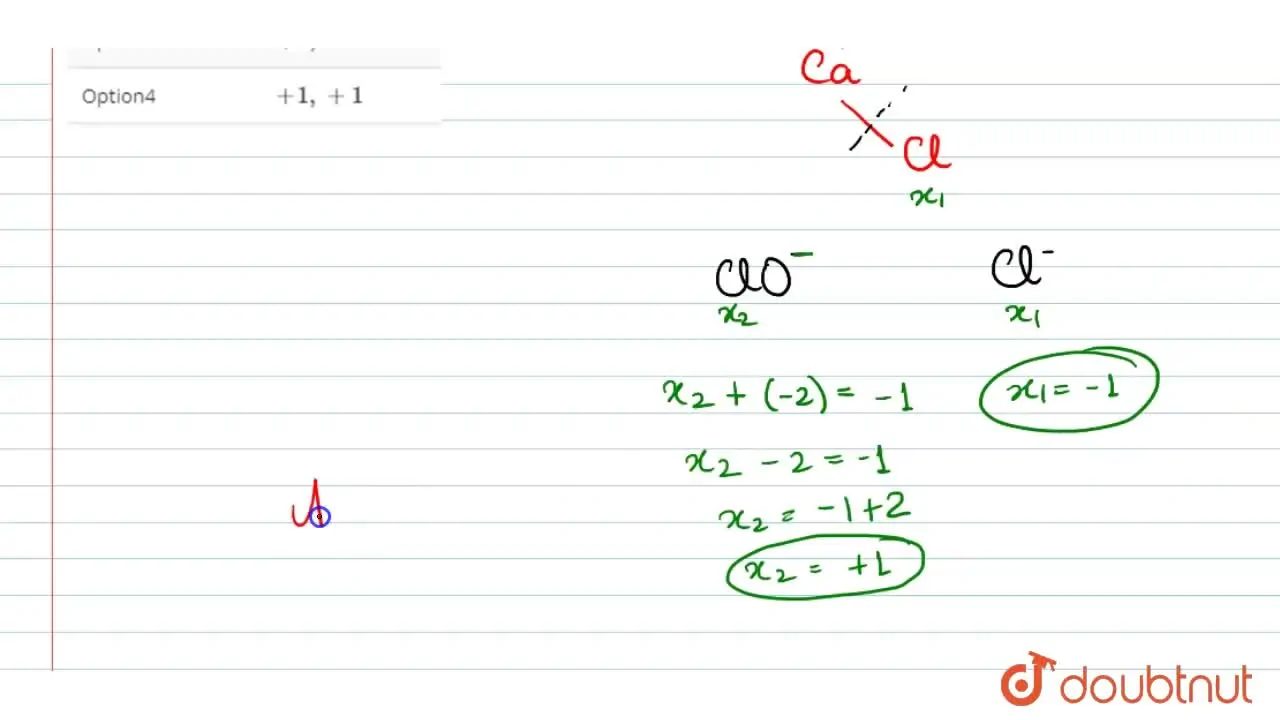Bleaching Powder Oxidation State Of Cl . The chemical formula of bleaching powder is as follows: The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Therefore, the correct answer is option c. Hence, option (c) is the correct answer. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. We should be knowing that. Oxidation state is $ +. The video explains the oxidation state(s) of chlorine in bleaching powder.
from www.doubtnut.com
In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The chemical formula of bleaching powder is as follows: Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Therefore, the correct answer is option c. Oxidation state is $ +. We should be knowing that. The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer.
In bleaching powder, the oxidation states of chlorine are
Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Therefore, the correct answer is option c. Oxidation state is $ +. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The video explains the oxidation state(s) of chlorine in bleaching powder. The chemical formula of bleaching powder is as follows: Hence, option (c) is the correct answer. We should be knowing that.
From www.youtube.com
Oxidation state(s) of chlorine in CaOCl_(2) (bleaching powder) 11 Bleaching Powder Oxidation State Of Cl Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. We should be knowing that. Oxidation state is $ +. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Hence, option (c) is the correct answer. Therefore, the correct answer is option c. The chemical formula of bleaching powder. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
The oxidation state of Cl in CaOCl2 bleaching powder is Shan Bleaching Powder Oxidation State Of Cl Oxidation state is $ +. The chemical formula of bleaching powder is as follows: The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Now we would determine the oxidation state in. Bleaching Powder Oxidation State Of Cl.
From www.doubtnut.com
The average oxidation state of chlorine in bleaching powder is Bleaching Powder Oxidation State Of Cl The video explains the oxidation state(s) of chlorine in bleaching powder. Therefore, the correct answer is option c. Hence, option (c) is the correct answer. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. We should be knowing that. The chemical formula of bleaching powder is as follows: In the. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
The oxidation number(s) of two \( \mathrm{Cl} \) atoms in bleaching Bleaching Powder Oxidation State Of Cl The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. We should be knowing that. The video explains the oxidation. Bleaching Powder Oxidation State Of Cl.
From www.doubtnut.com
In bleaching powder, the oxidation states of chlorine are Bleaching Powder Oxidation State Of Cl We should be knowing that. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The chemical formula of bleaching powder is as follows: The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. Oxidation state is $ +. The average oxidation. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
117. The oxidation state(s) of Cl in CaoCl2 (bleaching powder) is/are Bleaching Powder Oxidation State Of Cl In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. We should be knowing that. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The chemical formula of bleaching powder is as follows: Hence, option (c) is the correct answer. Oxidation state is $ +. Therefore, the correct. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
Determination of percentage of Available chlorine in a sample of Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Oxidation state is $ +. Therefore, the correct answer is option c. The chemical formula of bleaching powder is as follows: The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The. Bleaching Powder Oxidation State Of Cl.
From askfilo.com
Oxidation number of Cl in CaOCl2 (bleaching powder is) Filo Bleaching Powder Oxidation State Of Cl We should be knowing that. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Hence, option (c) is the correct answer. Therefore, the correct answer is option c. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ >. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
me in 49] Oxidation state of chlorine in Bleaching powder caoca 1)2 2 Bleaching Powder Oxidation State Of Cl The video explains the oxidation state(s) of chlorine in bleaching powder. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Therefore, the correct answer is option c. The chemical formula of bleaching powder is as follows: We should. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
Oxidation state of chlorine in Bleaching powder (CaOCl2)./Oxidation Bleaching Powder Oxidation State Of Cl The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. We should be knowing that. Hence, option (c) is the correct answer. The chemical formula of bleaching powder is as follows: Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. Therefore, the correct answer is option c. The video. Bleaching Powder Oxidation State Of Cl.
From powderbgh.blogspot.com
Oxidation Number Of Chlorine In Bleaching Powder POWDER BGH Bleaching Powder Oxidation State Of Cl The video explains the oxidation state(s) of chlorine in bleaching powder. Therefore, the correct answer is option c. Hence, option (c) is the correct answer. The chemical formula of bleaching powder is as follows: Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. Oxidation state is $ +. The average. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
Lec 14 Redox Reaction Oxidation number of each chlorine in Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. We should be knowing that. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The video explains the oxidation state(s) of chlorine in bleaching powder. Oxidation state is $ +. Therefore,. Bleaching Powder Oxidation State Of Cl.
From www.doubtnut.com
The oxidation of two Cl atoms in bleaching powder Ca(OCl)Cl is Bleaching Powder Oxidation State Of Cl Oxidation state is $ +. We should be knowing that. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. The chemical formula of bleaching powder is as follows: Now we would. Bleaching Powder Oxidation State Of Cl.
From www.numerade.com
SOLVEDThe oxidation state(s) of \mathrm{Cl} in \mathrm{CaOCl}_{2 Bleaching Powder Oxidation State Of Cl The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The chemical formula of bleaching powder is as follows: Therefore, the. Bleaching Powder Oxidation State Of Cl.
From www.academia.edu
(PDF) Expt. No 4 4 4 4 Determination of the Percentage of Available Bleaching Powder Oxidation State Of Cl We should be knowing that. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Therefore, the correct answer is option c. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The average oxidation state of $cl$ atom in $ca(ocl)cl$. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
How to Calculate Oxidation State of Ca, O, Cl in CaOCl₂(bleaching Bleaching Powder Oxidation State Of Cl In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Hence, option (c) is the correct answer. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. Oxidation state is $ +. The video explains the oxidation state(s) of chlorine in. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
Reaction of Bleaching Powder with Carbondioxide and Water, Chemistry Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Therefore, the correct answer is option c. The video explains the oxidation state(s) of chlorine in bleaching powder. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The chemical formula of. Bleaching Powder Oxidation State Of Cl.
From www.doubtnut.com
In bleaching powder, the oxidation states of chlorine are Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. Therefore, the correct answer is option c. We should be knowing that. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Oxidation state. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
isequaltoklasses What is the oxidation number of Chlorine in Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. The video explains the oxidation state(s) of chlorine in bleaching powder. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. Oxidation state is. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
Bleaching powder (CaOCl2) has two types of chlorine atoms with Bleaching Powder Oxidation State Of Cl Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. We should be knowing that. The chemical formula of bleaching powder is as follows: Hence, option (c) is the correct answer.. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
Test 4 (CodeD) 46. Consider the reaction 2Ca(OH)2 + 2Cl2 → Bleaching Powder Oxidation State Of Cl The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Oxidation state is $ +. We should be knowing that. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. The chemical. Bleaching Powder Oxidation State Of Cl.
From askfilo.com
Oxidation number of Cl in CaOCl2 (bleaching power) is Filo Bleaching Powder Oxidation State Of Cl The video explains the oxidation state(s) of chlorine in bleaching powder. We should be knowing that. Oxidation state is $ +. Hence, option (c) is the correct answer. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine. Bleaching Powder Oxidation State Of Cl.
From byjus.com
oxidation number of Both Cl in Ca(OCl)Cl Bleaching Powder Oxidation State Of Cl We should be knowing that. The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. In the case of bleaching powder (caocl_{2}),. Bleaching Powder Oxidation State Of Cl.
From www.pinterest.com
Bleaching Powder Bleaching powder, Chemical structure, Molar mass Bleaching Powder Oxidation State Of Cl Therefore, the correct answer is option c. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Oxidation state is $ +. Hence, option (c) is the correct answer. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ >. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
Bleaching powder (CaOCl2) has two types of chlorine atoms with Bleaching Powder Oxidation State Of Cl Therefore, the correct answer is option c. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The chemical formula of bleaching powder is as follows: We should be knowing that. Hence, option (c) is the correct answer. Oxidation state. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
Oxidation number of chlorine atoms in Bleaching powder(CaOCl2) YouTube Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. We should be knowing that. The chemical formula of bleaching powder is as follows: The video explains the oxidation state(s) of chlorine in bleaching powder. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The average oxidation state of $cl$ atom in. Bleaching Powder Oxidation State Of Cl.
From www.youtube.com
The average oxidation state of chlorine in bleaching powder is CLASS Bleaching Powder Oxidation State Of Cl Therefore, the correct answer is option c. The chemical formula of bleaching powder is as follows: We should be knowing that. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The video explains the oxidation state(s) of chlorine in bleaching powder. Hence, option (c) is the correct answer. Oxidation state. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
Bleaching powder is obtained by the action of chlorine gas and Bleaching Powder Oxidation State Of Cl The chemical formula of bleaching powder is as follows: In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. The video explains the oxidation state(s) of chlorine in bleaching powder. We should be knowing that. Therefore, the correct answer. Bleaching Powder Oxidation State Of Cl.
From www.doubtnut.com
The possible oxidation state of two chlorine atoms in bleaching powder Bleaching Powder Oxidation State Of Cl The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Oxidation state is $ +. Therefore, the correct answer is option c. We should be knowing that. The video explains the oxidation state(s) of chlorine in bleaching powder. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The chemical. Bleaching Powder Oxidation State Of Cl.
From www.toppr.com
Oxidation number of Cl in CaOCl2 (bleaching powder) is Bleaching Powder Oxidation State Of Cl In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. The chemical formula of bleaching powder is as follows: We should be knowing that. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Therefore, the correct answer is option c. The video explains the oxidation state(s) of chlorine. Bleaching Powder Oxidation State Of Cl.
From askfilo.com
Oxidation number of Cl atoms in CaOCl2 (bleaching powder) Filo Bleaching Powder Oxidation State Of Cl Therefore, the correct answer is option c. The chemical formula of bleaching powder is as follows: Hence, option (c) is the correct answer. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Now we would determine the oxidation. Bleaching Powder Oxidation State Of Cl.
From www.doubtnut.com
In bleaching powder, the oxidation states of chlorine are Bleaching Powder Oxidation State Of Cl Oxidation state is $ +. We should be knowing that. Therefore, the correct answer is option c. In the case of bleaching powder (caocl_{2}), the individual oxidation states of chlorine can be calculated by first understanding the. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The video explains the. Bleaching Powder Oxidation State Of Cl.
From askfilo.com
The oxidation number of two Cl atoms in bleaching powder is/are Filo Bleaching Powder Oxidation State Of Cl The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Hence, option (c) is the correct answer. The chemical formula of bleaching powder is as follows: Oxidation state is $ +. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The video explains the oxidation state(s) of chlorine in. Bleaching Powder Oxidation State Of Cl.
From www.scribd.com
Determination of Available Chlorine in Bleaching Powder PDF Bleach Bleaching Powder Oxidation State Of Cl The video explains the oxidation state(s) of chlorine in bleaching powder. The chemical formula of bleaching powder is as follows: We should be knowing that. Therefore, the correct answer is option c. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. Oxidation state is $ +. Hence, option (c) is the correct answer. In the case of bleaching. Bleaching Powder Oxidation State Of Cl.
From byjus.com
the average oxidation of chlorine in bleaching powder is Bleaching Powder Oxidation State Of Cl Hence, option (c) is the correct answer. We should be knowing that. The chemical formula of bleaching powder is as follows: Therefore, the correct answer is option c. Now we would determine the oxidation state in $ca{o_2}c{l_2}$ > bleaching powder has two types of cl atom which. The average oxidation state of $cl$ atom in $ca(ocl)cl$ is zero. In the. Bleaching Powder Oxidation State Of Cl.