Heat Capacity And Calorimetry . Additionally, it can find the. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. Learn about different types of calorimeters, the calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. The specific heat is numerically equal to the amount of heat necessary to. The calorimetry calculator can help you solve complex calorimetry problems. It can analyze the heat exchange between up to 3 objects. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Apply the first law of thermodynamics to calorimetry ; Calorimetry is used to measure amounts of heat transferred to or. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the.
from www.slideshare.net
Learn about different types of calorimeters, the calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. The calorimetry calculator can help you solve complex calorimetry problems. The specific heat is numerically equal to the amount of heat necessary to. Calorimetry is used to measure amounts of heat transferred to or. It can analyze the heat exchange between up to 3 objects.
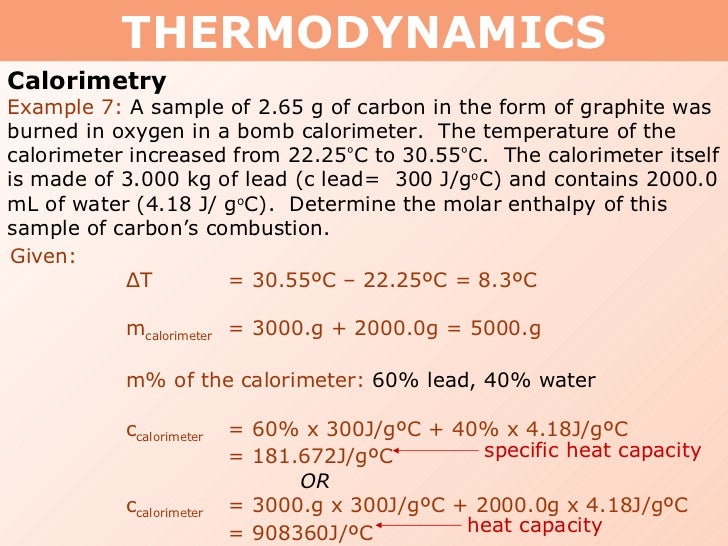
Tang 01 heat capacity and calorimetry
Heat Capacity And Calorimetry Additionally, it can find the. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. The specific heat is numerically equal to the amount of heat necessary to. It can analyze the heat exchange between up to 3 objects. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The calorimetry calculator can help you solve complex calorimetry problems. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Additionally, it can find the. Calorimetry is used to measure amounts of heat transferred to or. Apply the first law of thermodynamics to calorimetry ; Learn about different types of calorimeters, the calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity And Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Apply the first law of thermodynamics to calorimetry ; The heat capacity of the calorimeter or of the reaction mixture may be used to. Heat Capacity And Calorimetry.
From www.youtube.com
Thermochemistry Heat Capacity and Calorimetry YouTube Heat Capacity And Calorimetry Learn about different types of calorimeters, the calorimetry. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The calorimetry calculator can help you solve complex calorimetry problems. It can analyze. Heat Capacity And Calorimetry.
From cernvbyt.blob.core.windows.net
Can Heat Capacity Of Calorimeter Be Negative at Lulu Seaton blog Heat Capacity And Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure amounts of heat transferred to or. Apply the first. Heat Capacity And Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity And Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. The specific heat is numerically equal to the amount of heat necessary to. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Heat Capacity And Calorimetry.
From www.youtube.com
Heat Capacity, Specific Heat, and Calorimetry YouTube Heat Capacity And Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. Additionally, it can find the. Apply the first law. Heat Capacity And Calorimetry.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Heat Capacity And Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Learn about different types of calorimeters, the calorimetry. Apply. Heat Capacity And Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity And Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. The specific heat is numerically equal to the amount of heat necessary to. It can analyze the heat exchange between up to 3 objects. The symbol c stands for the specific heat (also called. Heat Capacity And Calorimetry.
From www.youtube.com
CH5 Q5 Calculating the Heat Capacity of a Calorimeter YouTube Heat Capacity And Calorimetry Additionally, it can find the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. It can analyze the heat exchange between up to 3 objects. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. Heat Capacity And Calorimetry.
From studylib.net
Calorimetry Lab Specific Heat Capacity Heat Capacity And Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. One technique we can use to measure the amount of heat involved in a chemical or physical process. Heat Capacity And Calorimetry.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Heat Capacity And Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. The heat capacity of the calorimeter or of. Heat Capacity And Calorimetry.
From www.youtube.com
Class 11th Physics Specific Heat Capacity Calorimetry Example 10. Heat Capacity And Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This is a typical calorimetry problem—two bodies at different temperatures are brought in. Heat Capacity And Calorimetry.
From www.youtube.com
Principle of Calorimetry YouTube Heat Capacity And Calorimetry It can analyze the heat exchange between up to 3 objects. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Apply the first law of thermodynamics to calorimetry ; This is a typical calorimetry. Heat Capacity And Calorimetry.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Heat Capacity And Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. One technique we can use to measure the amount of heat involved in. Heat Capacity And Calorimetry.
From www.slideserve.com
PPT CHEM 1011 PowerPoint Presentation, free download ID317228 Heat Capacity And Calorimetry Learn about different types of calorimeters, the calorimetry. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It can analyze the heat exchange between up to 3 objects. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or. Heat Capacity And Calorimetry.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Heat Capacity And Calorimetry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Apply the first law of thermodynamics to calorimetry ; One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. This is a typical calorimetry problem—two bodies at different temperatures. Heat Capacity And Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity And Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. It can analyze the. Heat Capacity And Calorimetry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Heat Capacity And Calorimetry The specific heat is numerically equal to the amount of heat necessary to. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The heat capacity of the calorimeter or of the reaction mixture. Heat Capacity And Calorimetry.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Heat Capacity And Calorimetry Learn about different types of calorimeters, the calorimetry. The specific heat is numerically equal to the amount of heat necessary to. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. Apply the first law of thermodynamics to calorimetry ; It can analyze the. Heat Capacity And Calorimetry.
From chem.libretexts.org
7.3 Heats of Reactions and Calorimetry Chemistry LibreTexts Heat Capacity And Calorimetry Additionally, it can find the. Learn about different types of calorimeters, the calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. Apply the first law of. Heat Capacity And Calorimetry.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID4499939 Heat Capacity And Calorimetry The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the chemical. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact. Heat Capacity And Calorimetry.
From www.youtube.com
050 Calorimetry YouTube Heat Capacity And Calorimetry The specific heat is numerically equal to the amount of heat necessary to. Calorimetry is used to measure amounts of heat transferred to or. The calorimetry calculator can help you solve complex calorimetry problems. The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. One technique we can use to. Heat Capacity And Calorimetry.
From slideplayer.com
Specific Heat Capacity & Calorimetry ppt download Heat Capacity And Calorimetry The symbol c stands for the specific heat (also called “specific heat capacity”) and depends on the material and phase. The specific heat is numerically equal to the amount of heat necessary to. Apply the first law of thermodynamics to calorimetry ; The calorimetry calculator can help you solve complex calorimetry problems. Learn about different types of calorimeters, the calorimetry.. Heat Capacity And Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity And Calorimetry Apply the first law of thermodynamics to calorimetry ; It can analyze the heat exchange between up to 3 objects. Calorimetry is used to measure amounts of heat transferred to or. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat capacity of the calorimeter or. Heat Capacity And Calorimetry.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Heat Capacity And Calorimetry Apply the first law of thermodynamics to calorimetry ; The specific heat is numerically equal to the amount of heat necessary to. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by the. The calorimetry calculator can help you solve complex calorimetry problems. Learn about different. Heat Capacity And Calorimetry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Heat Capacity And Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. Calorimetry is used to measure amounts of heat transferred to or. Apply the first law of thermodynamics to calorimetry ; The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. Heat Capacity And Calorimetry.
From studylib.net
Calorimetry_and_Specific_Heat_Capacity Heat Capacity And Calorimetry It can analyze the heat exchange between up to 3 objects. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed. Heat Capacity And Calorimetry.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Heat Capacity And Calorimetry The specific heat is numerically equal to the amount of heat necessary to. Apply the first law of thermodynamics to calorimetry ; Calorimetry is used to measure amounts of heat transferred to or. Learn about different types of calorimeters, the calorimetry. It can analyze the heat exchange between up to 3 objects. The calorimetry calculator can help you solve complex. Heat Capacity And Calorimetry.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Heat Capacity And Calorimetry This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat until a common temperature is reached. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Heat Capacity And Calorimetry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Heat Capacity And Calorimetry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. It can analyze the heat exchange between up to 3 objects. The specific heat is numerically equal to the amount of heat necessary to. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other. Heat Capacity And Calorimetry.
From www.chegg.com
Solved connect Calorimetry TRY. HEAT Heat Capacity And Calorimetry Apply the first law of thermodynamics to calorimetry ; Calorimetry is used to measure amounts of heat transferred to or. The specific heat is numerically equal to the amount of heat necessary to. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The calorimetry calculator can help you solve complex. Heat Capacity And Calorimetry.
From www.slideshare.net
Tang 01 heat capacity and calorimetry Heat Capacity And Calorimetry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is used to measure amounts of heat transferred to or. The calorimetry calculator can help you solve complex calorimetry problems. This is a typical calorimetry problem—two bodies at different temperatures are brought in contact with each other and exchange heat. Heat Capacity And Calorimetry.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube Heat Capacity And Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. Learn about different types of calorimeters, the calorimetry. Calorimetry is used to measure amounts of heat transferred to or. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of heat released or absorbed by. Heat Capacity And Calorimetry.
From www.youtube.com
Specific Heat Capacity Calorimetry AP Physics 2 and AP Chemistry Heat Capacity And Calorimetry The calorimetry calculator can help you solve complex calorimetry problems. Apply the first law of thermodynamics to calorimetry ; One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat capacity of the calorimeter or of the reaction mixture may be used to calculate the amount of. Heat Capacity And Calorimetry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Heat Capacity And Calorimetry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter ;. Learn about different types of calorimeters, the calorimetry. Apply the first law of thermodynamics to calorimetry ; It can analyze the heat exchange between. Heat Capacity And Calorimetry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Heat Capacity And Calorimetry One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It can analyze the heat exchange between up to 3 objects. Learn about different types of calorimeters, the calorimetry. Apply the first law of thermodynamics to calorimetry ; The symbol c stands for the specific heat (also called. Heat Capacity And Calorimetry.