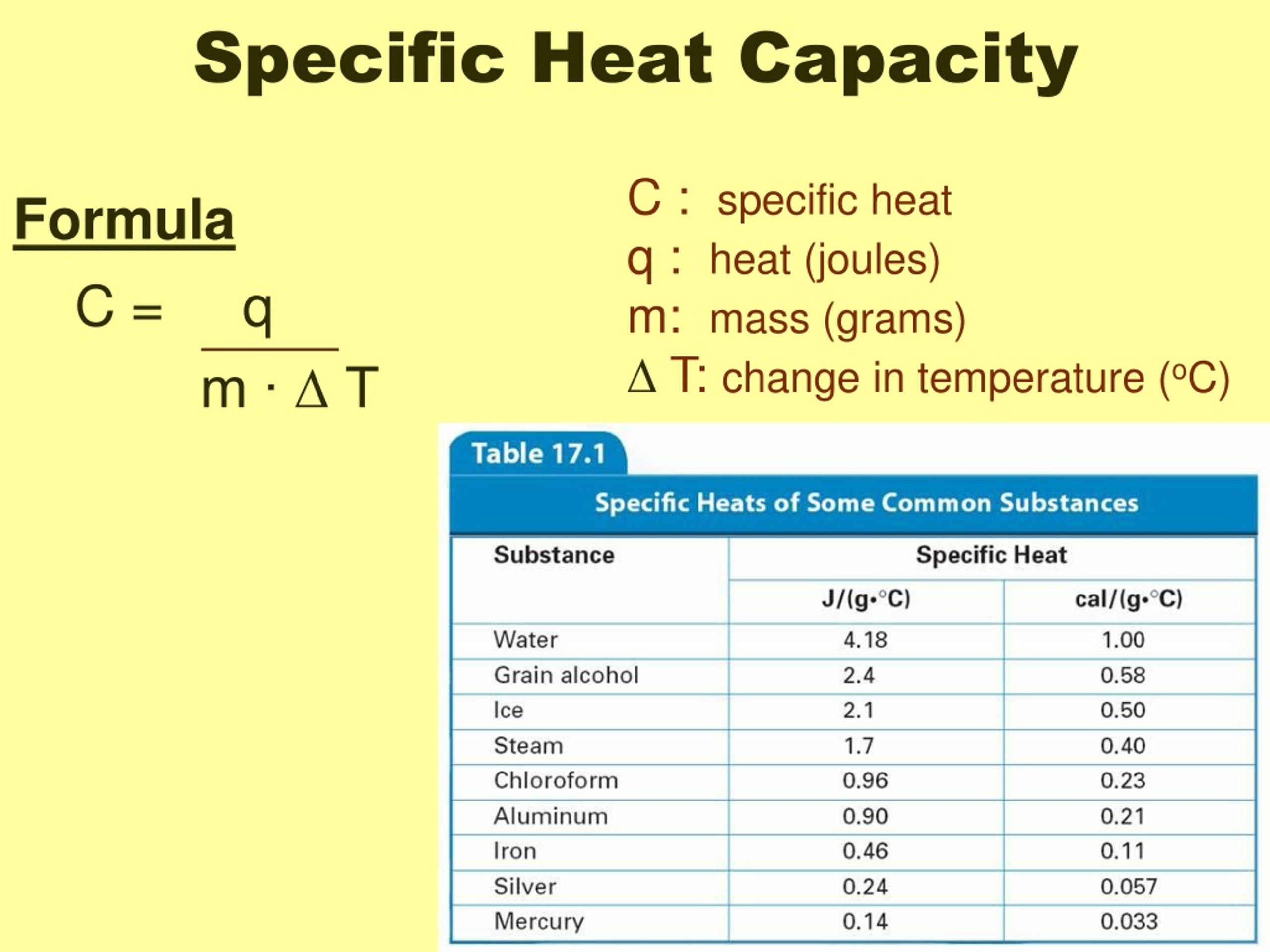
What Is Specific Heat Capacity In Thermodynamics . Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. In equation form, heat capacity c is c = m c,. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. Specific heat is the heat capacity in calories per gram. It describes how much heat must be added to a unit of mass of a given substance to raise its. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It may also be expressed as j/kg·k. The calorie is defined by the specific heat of water, which is defined as one calorie per degree. In si units, specific heat capacity (symbol: Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It plays a crucial role in understanding how.
from www.slideserve.com
Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It may also be expressed as j/kg·k. Specific heat is the heat capacity in calories per gram. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). In si units, specific heat capacity (symbol: It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. It plays a crucial role in understanding how. The calorie is defined by the specific heat of water, which is defined as one calorie per degree. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume.
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free
What Is Specific Heat Capacity In Thermodynamics Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Specific heat is the heat capacity in calories per gram. The calorie is defined by the specific heat of water, which is defined as one calorie per degree. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It plays a crucial role in understanding how. In si units, specific heat capacity (symbol: Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It may also be expressed as j/kg·k. It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. In equation form, heat capacity c is c = m c,. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It describes how much heat must be added to a unit of mass of a given substance to raise its.
From scienceinfo.com
Specific Heat Capacity Definition, Unit, Formula What Is Specific Heat Capacity In Thermodynamics It may also be expressed as j/kg·k. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Specific heat is the heat capacity in calories per gram. It is expressed in joules per gram. What Is Specific Heat Capacity In Thermodynamics.
From www.slidemake.com
Einstein Theory Of Specific Heat Presentation What Is Specific Heat Capacity In Thermodynamics It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Specific heat is. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT AP Chemistry Unit 7 Thermodynamics PowerPoint Presentation What Is Specific Heat Capacity In Thermodynamics It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. It plays a crucial role in understanding how. In equation form, heat capacity c is c = m c,. It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram. What Is Specific Heat Capacity In Thermodynamics.
From studymind.co.uk
Heat Capacity Study Mind What Is Specific Heat Capacity In Thermodynamics The calorie is defined by the specific heat of water, which is defined as one calorie per degree. It may also be expressed as j/kg·k. Specific heat is the heat capacity in calories per gram. It describes how much heat must be added to a unit of mass of a given substance to raise its. Heat capacity is the amount. What Is Specific Heat Capacity In Thermodynamics.
From www.geeksforgeeks.org
Specific Heat Capacity Definition, Formula, Water Heat Capacity What Is Specific Heat Capacity In Thermodynamics In equation form, heat capacity c is c = m c,. It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. In si units, specific heat capacity (symbol: It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT CHAPTER 16 THERMODYNAMICS PowerPoint Presentation, free What Is Specific Heat Capacity In Thermodynamics Specific heat is the heat capacity in calories per gram. In si units, specific heat capacity (symbol: Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. In equation form, heat capacity c is c = m c,. Unlike the total heat capacity, the specific heat capacity is independent of mass or. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT Advance Chemical Engineering Thermodynamics PowerPoint What Is Specific Heat Capacity In Thermodynamics Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It plays a crucial role. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT Chapter 17 Thermochemistry PowerPoint Presentation, free What Is Specific Heat Capacity In Thermodynamics Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). In si units, specific heat capacity (symbol: It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. In equation form, heat capacity c is c = m. What Is Specific Heat Capacity In Thermodynamics.
From www.tes.com
GCSE Physics Specific Heat Capacity Teaching Resources What Is Specific Heat Capacity In Thermodynamics The calorie is defined by the specific heat of water, which is defined as one calorie per degree. In si units, specific heat capacity (symbol: It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific heat is defined as the amount of heat required to raise the temperature of. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube What Is Specific Heat Capacity In Thermodynamics In si units, specific heat capacity (symbol: C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It describes how much heat must be added to a unit of mass. What Is Specific Heat Capacity In Thermodynamics.
From haipernews.com
How To Calculate Specific Heat Capacity Bbc Bitesize Haiper What Is Specific Heat Capacity In Thermodynamics Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. In si units, specific heat capacity (symbol: The calorie is defined by the specific heat of water, which is defined as one calorie per degree. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. C) is. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT Chapters 19, 20 Temperature, Heat, and the First Law of What Is Specific Heat Capacity In Thermodynamics The calorie is defined by the specific heat of water, which is defined as one calorie per degree. Specific heat is the heat capacity in calories per gram. It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Unlike the total heat capacity, the specific. What Is Specific Heat Capacity In Thermodynamics.
From study.com
Specific Heat Capacity Definition, Formula & Calculation Video What Is Specific Heat Capacity In Thermodynamics In si units, specific heat capacity (symbol: It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. In equation form, heat capacity c is c = m c,. The calorie is defined by the specific heat of water, which is defined as one calorie per. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube What Is Specific Heat Capacity In Thermodynamics In si units, specific heat capacity (symbol: In equation form, heat capacity c is c = m c,. It describes how much heat must be added to a unit of mass of a given substance to raise its. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It plays a crucial. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances What Is Specific Heat Capacity In Thermodynamics It describes how much heat must be added to a unit of mass of a given substance to raise its. It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. C). What Is Specific Heat Capacity In Thermodynamics.
From thermalengineeringlearn.blogspot.com
THERMAL ENGINEERING Thermodynamic Equilibrium What Is Specific Heat Capacity In Thermodynamics It describes how much heat must be added to a unit of mass of a given substance to raise its. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. It plays a crucial. What Is Specific Heat Capacity In Thermodynamics.
From www.tes.com
Specific heat capacity complete lesson (GCSE 19) Teaching Resources What Is Specific Heat Capacity In Thermodynamics In equation form, heat capacity c is c = m c,. It may also be expressed as j/kg·k. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). In si units, specific heat capacity (symbol: Heat capacity is the amount of heat necessary to change the temperature of. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Specific heat capacity YouTube What Is Specific Heat Capacity In Thermodynamics It describes how much heat must be added to a unit of mass of a given substance to raise its. In equation form, heat capacity c is c = m c,. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). Unlike the total heat capacity, the specific. What Is Specific Heat Capacity In Thermodynamics.
From classnotes.org.in
Heat Capacity Chemistry, Class 11, Thermodynamics What Is Specific Heat Capacity In Thermodynamics The calorie is defined by the specific heat of water, which is defined as one calorie per degree. In equation form, heat capacity c is c = m c,. In si units, specific heat capacity (symbol: It describes how much heat must be added to a unit of mass of a given substance to raise its. Specific heat capacity is. What Is Specific Heat Capacity In Thermodynamics.
From www.grc.nasa.gov
Specific Heats What Is Specific Heat Capacity In Thermodynamics In si units, specific heat capacity (symbol: Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. In equation form, heat capacity c is c = m c,. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Thermodynamics Specific Heat Capacity Calculations YouTube What Is Specific Heat Capacity In Thermodynamics In equation form, heat capacity c is c = m c,. It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. The calorie is defined by the specific heat of water, which is defined as one calorie per degree. It plays a crucial role in understanding how. Specific heat. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial What Is Specific Heat Capacity In Thermodynamics In si units, specific heat capacity (symbol: It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. Specific heat is the heat capacity in. What Is Specific Heat Capacity In Thermodynamics.
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec What Is Specific Heat Capacity In Thermodynamics It describes how much heat must be added to a unit of mass of a given substance to raise its. It plays a crucial role in understanding how. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). Heat capacity is the amount of heat necessary to change. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
CHEMISTRY 101 Specific heat capacity and calculating heat YouTube What Is Specific Heat Capacity In Thermodynamics In si units, specific heat capacity (symbol: C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. In equation form, heat capacity c is c = m c,. It describes how much heat must be added to a unit of mass of a given substance to raise its. Unlike the total heat. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT Heat capacity and Specific Heat PowerPoint Presentation, free What Is Specific Heat Capacity In Thermodynamics C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. In equation form, heat capacity c is c = m c,. It plays a crucial role in understanding how. Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one. What Is Specific Heat Capacity In Thermodynamics.
From engineerexcel.com
Specific Heat vs Heat Capacity Essential Thermodynamic Concepts What Is Specific Heat Capacity In Thermodynamics Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It describes how much heat must be added to a unit of mass of a given substance to raise its. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. It plays a crucial role in understanding. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Specific Heat Capacity Example Problem Physics YouTube What Is Specific Heat Capacity In Thermodynamics Specific heat is defined as the amount of heat required to raise the temperature of a unit mass of a substance by one degree celsius. It may also be expressed as j/kg·k. C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. In si units, specific heat capacity (symbol: The calorie is. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Specific Heat and Heat Capacity YouTube What Is Specific Heat Capacity In Thermodynamics It may also be expressed as j/kg·k. In equation form, heat capacity c is c = m c,. It plays a crucial role in understanding how. The calorie is defined by the specific heat of water, which is defined as one calorie per degree. Specific heat is defined as the amount of heat required to raise the temperature of a. What Is Specific Heat Capacity In Thermodynamics.
From studylib.net
1.3 Specific Heat Capacity What Is Specific Heat Capacity In Thermodynamics Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It describes how much heat must be added to a unit of mass of a given substance to raise its. In equation form, heat capacity c is c = m c,. It may also be expressed as j/kg·k.. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi What Is Specific Heat Capacity In Thermodynamics Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It plays a crucial role in understanding how. It describes how much heat must be added to a unit of. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
Specific Heat capacities of gas Cp and Cv First law of What Is Specific Heat Capacity In Thermodynamics Specific heat is the heat capacity in calories per gram. It is expressed in joules per gram per degree celsius (j g×oc j g × o c), and is given by. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). Unlike the total heat capacity, the specific. What Is Specific Heat Capacity In Thermodynamics.
From www.slideserve.com
PPT 18 Heat and the First Law of Thermodynamics PowerPoint What Is Specific Heat Capacity In Thermodynamics In equation form, heat capacity c is c = m c,. Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). The calorie is defined by the specific heat of water, which is defined as one calorie per degree. In si units, specific heat capacity (symbol: C) is. What Is Specific Heat Capacity In Thermodynamics.
From www.worksheetsplanet.com
What is Specific Heat What Is Specific Heat Capacity In Thermodynamics C) is the amount of heat in joules required to raise 1 gram of a substance 1 kelvin. In equation form, heat capacity c is c = m c,. Unlike the total heat capacity, the specific heat capacity is independent of mass or volume. It may also be expressed as j/kg·k. The calorie is defined by the specific heat of. What Is Specific Heat Capacity In Thermodynamics.
From www.youtube.com
What Is The Difference Between Specific Heat Capacity, Heat Capacity What Is Specific Heat Capacity In Thermodynamics Specific heat capacity is the amount of heat needed to raise one gram of a material by one degree celsius (o c). It may also be expressed as j/kg·k. It plays a crucial role in understanding how. Specific heat is the heat capacity in calories per gram. In si units, specific heat capacity (symbol: Specific heat is defined as the. What Is Specific Heat Capacity In Thermodynamics.
From physics.stackexchange.com
thermodynamics Derivation of heat capacity at constant pressure and What Is Specific Heat Capacity In Thermodynamics It may also be expressed as j/kg·k. Heat capacity is the amount of heat necessary to change the temperature of a substance by 1.00 °c. Specific heat is the heat capacity in calories per gram. It plays a crucial role in understanding how. In si units, specific heat capacity (symbol: It is expressed in joules per gram per degree celsius. What Is Specific Heat Capacity In Thermodynamics.