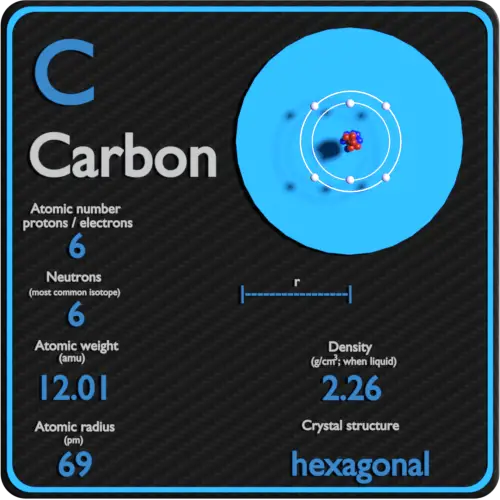
How Many Energy Levels Does Carbon Has . How many energy levels does carbon have? The remaining two electrons occupy the 2p subshell. The p orbital exists in energy level 3 and up. Four of them fill the 1s and 2s orbitals. For excited states, the most typical. Carbon has 2 energy levels. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. We now have a choice of filling one of. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. Carbon (atomic number 6) has six electrons. Carbon (atomic number 6) has six electrons. 19 rows two electrons completely fill the first energy level. In its neutral state, carbon has 6. This table shows the pattern in the periodic table that mendeleev developed and. The remaining two electrons occupy the 2p subshell.
from material-properties.org
In its neutral state, carbon has 6. Carbon (atomic number 6) has six electrons. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. This table shows the pattern in the periodic table that mendeleev developed and. The p orbital exists in energy level 3 and up. Four of them fill the 1s and 2s orbitals. True/false give the spectroscopic notation (also called. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. We now have a choice of filling one of. Carbon has 2 energy levels.
Carbon Periodic Table and Atomic Properties
How Many Energy Levels Does Carbon Has The p orbital exists in energy level 3 and up. Carbon has 2 energy levels. 116 rows electrons orbit the atom's nucleus in energy levels. Carbon (atomic number 6) has six electrons. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. The remaining two electrons occupy the 2p subshell. This table shows the pattern in the periodic table that mendeleev developed and. The first energy level can hold up to 2 electrons, and the second can hold up to 8. We now have a choice of filling one of. True/false give the spectroscopic notation (also called. For excited states, the most typical. The remaining two electrons occupy the 2p subshell. Four of them fill the 1s and 2s orbitals. In its neutral state, carbon has 6. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. How many energy levels does carbon have?
From pixels.com
Carbon Electron Configuration Photograph by Photo How Many Energy Levels Does Carbon Has 19 rows two electrons completely fill the first energy level. In its neutral state, carbon has 6. The first energy level can hold up to 2 electrons, and the second can hold up to 8. 116 rows electrons orbit the atom's nucleus in energy levels. How many energy levels does carbon have? Carbon (atomic number 6) has six electrons. Carbon. How Many Energy Levels Does Carbon Has.
From www.alamy.com
A carbon atom, with mass and energy levels. Vector illustration Stock How Many Energy Levels Does Carbon Has 116 rows electrons orbit the atom's nucleus in energy levels. The first energy level can hold up to 2 electrons, and the second can hold up to 8. In its neutral state, carbon has 6. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. 19 rows two electrons completely. How Many Energy Levels Does Carbon Has.
From chemistry291.blogspot.com
5 Steps】How Many Valence Electrons Does Carbon Have?Number of Valence How Many Energy Levels Does Carbon Has In its neutral state, carbon has 6. We now have a choice of filling one of. 19 rows two electrons completely fill the first energy level. Carbon (atomic number 6) has six electrons. The remaining two electrons occupy the 2p subshell. The remaining two electrons occupy the 2p subshell. Carbon has 2 energy levels. 116 rows electrons orbit the atom's. How Many Energy Levels Does Carbon Has.
From ascensionglossary.com
Carbon Atom Ascension Glossary How Many Energy Levels Does Carbon Has True/false give the spectroscopic notation (also called. We now have a choice of filling one of. The first energy level can hold up to 2 electrons, and the second can hold up to 8. Carbon (atomic number 6) has six electrons. How many energy levels does carbon have? 19 rows two electrons completely fill the first energy level. Because the. How Many Energy Levels Does Carbon Has.
From www.slideserve.com
PPT Major Organic Compounds PowerPoint Presentation, free download How Many Energy Levels Does Carbon Has For excited states, the most typical. Four of them fill the 1s and 2s orbitals. The first energy level can hold up to 2 electrons, and the second can hold up to 8. True/false give the spectroscopic notation (also called. Four of them fill the 1s and 2s orbitals. Carbon (atomic number 6) has six electrons. The ground state electron. How Many Energy Levels Does Carbon Has.
From valenceelectrons.com
How to Find the Valence Electrons for Carbon(C)? How Many Energy Levels Does Carbon Has Carbon has 2 energy levels. How many energy levels does carbon have? In its neutral state, carbon has 6. Four of them fill the 1s and 2s orbitals. The p orbital exists in energy level 3 and up. For excited states, the most typical. The remaining two electrons occupy the 2p subshell. True/false give the spectroscopic notation (also called. We. How Many Energy Levels Does Carbon Has.
From agrocorrn.com
The carbon cycle what it is, how it works and its importance AgroCorrn How Many Energy Levels Does Carbon Has For excited states, the most typical. The first energy level can hold up to 2 electrons, and the second can hold up to 8. How many energy levels does carbon have? In its neutral state, carbon has 6. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. This table shows the pattern in the periodic. How Many Energy Levels Does Carbon Has.
From mavink.com
Periodic Table With Energy Levels How Many Energy Levels Does Carbon Has The first energy level can hold up to 2 electrons, and the second can hold up to 8. This table shows the pattern in the periodic table that mendeleev developed and. The remaining two electrons occupy the 2p subshell. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. The p orbital exists. How Many Energy Levels Does Carbon Has.
From e360.yale.edu
How the World Passed a Carbon Threshold and Why It Matters Yale E360 How Many Energy Levels Does Carbon Has The p orbital exists in energy level 3 and up. Four of them fill the 1s and 2s orbitals. 19 rows two electrons completely fill the first energy level. Carbon has 2 energy levels. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. Carbon (atomic number 6) has six electrons. Carbon (atomic. How Many Energy Levels Does Carbon Has.
From www.stonecoldhands.com
Electron Shells and Orbitals Stone Cold Chemistry Talk How Many Energy Levels Does Carbon Has Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. This table shows the pattern in the periodic table that mendeleev developed and. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. The first energy level can hold up to 2. How Many Energy Levels Does Carbon Has.
From www.slideserve.com
PPT Ch 5 Atomic Structure and the Periodic Table PowerPoint How Many Energy Levels Does Carbon Has Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Carbon (atomic number 6) has six electrons. We now have a choice of filling one of. The p orbital exists in energy level 3 and up. True/false give the spectroscopic notation (also called. In its neutral state, carbon has 6.. How Many Energy Levels Does Carbon Has.
From manualdataoddfellows.z21.web.core.windows.net
Schematic Energy Level Diagram How Many Energy Levels Does Carbon Has For excited states, the most typical. Carbon (atomic number 6) has six electrons. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. Four of them fill the 1s and 2s orbitals. In its neutral state, carbon has 6. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the. How Many Energy Levels Does Carbon Has.
From www.elephango.com
All About Atoms Educational Resources K12 Learning, Chemistry, Science How Many Energy Levels Does Carbon Has Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. In its neutral state, carbon has 6. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. Carbon (atomic. How Many Energy Levels Does Carbon Has.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy How Many Energy Levels Does Carbon Has Carbon (atomic number 6) has six electrons. True/false give the spectroscopic notation (also called. The remaining two electrons occupy the 2p subshell. The p orbital exists in energy level 3 and up. Carbon has 2 energy levels. Carbon (atomic number 6) has six electrons. 116 rows electrons orbit the atom's nucleus in energy levels. The remaining two electrons occupy the. How Many Energy Levels Does Carbon Has.
From www.teachoo.com
[Class 10 Chemistry] What is Carbon and its compounds? Teachoo How Many Energy Levels Does Carbon Has Carbon (atomic number 6) has six electrons. 19 rows two electrons completely fill the first energy level. True/false give the spectroscopic notation (also called. 116 rows electrons orbit the atom's nucleus in energy levels. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. In its neutral state, carbon has 6. Carbon has 2 energy levels.. How Many Energy Levels Does Carbon Has.
From www.slideserve.com
PPT Chapter 71 Electrons and their Energy Levels Part 2 PowerPoint How Many Energy Levels Does Carbon Has The first energy level can hold up to 2 electrons, and the second can hold up to 8. Carbon (atomic number 6) has six electrons. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. In its neutral state, carbon has 6. How many energy levels does carbon have? 116. How Many Energy Levels Does Carbon Has.
From slideplayer.com
Bohr Model Energy levels ppt download How Many Energy Levels Does Carbon Has The p orbital exists in energy level 3 and up. How many energy levels does carbon have? Carbon (atomic number 6) has six electrons. 116 rows electrons orbit the atom's nucleus in energy levels. 19 rows two electrons completely fill the first energy level. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where. How Many Energy Levels Does Carbon Has.
From material-properties.org
Carbon Periodic Table and Atomic Properties How Many Energy Levels Does Carbon Has Carbon has 2 energy levels. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. The remaining two electrons occupy the 2p subshell. We now have a choice of filling one of. True/false give the spectroscopic notation (also called. In its neutral state, carbon has 6. 116 rows electrons orbit. How Many Energy Levels Does Carbon Has.
From www.pci.tu-bs.de
From Carbon to Neon How Many Energy Levels Does Carbon Has Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. The remaining two electrons occupy the 2p subshell. The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. Carbon (atomic number 6) has. How Many Energy Levels Does Carbon Has.
From www.alamy.com
Element carbon hires stock photography and images Alamy How Many Energy Levels Does Carbon Has How many energy levels does carbon have? Carbon (atomic number 6) has six electrons. True/false give the spectroscopic notation (also called. The p orbital exists in energy level 3 and up. Carbon (atomic number 6) has six electrons. We now have a choice of filling one of. The remaining two electrons occupy the 2p subshell. Carbon has 2 energy levels.. How Many Energy Levels Does Carbon Has.
From ar.inspiredpencil.com
Atom Energy Level Diagram How Many Energy Levels Does Carbon Has Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. For excited states, the most typical. The first energy level can hold up to 2 electrons, and the second can hold up to 8. In its neutral state, carbon has 6. We now have a choice of filling one of. Four of them fill. How Many Energy Levels Does Carbon Has.
From www.iqsdirectory.com
CO2 Lasers Types, Uses, Features and Benefits How Many Energy Levels Does Carbon Has Four of them fill the 1s and 2s orbitals. For excited states, the most typical. In its neutral state, carbon has 6. True/false give the spectroscopic notation (also called. The remaining two electrons occupy the 2p subshell. The first energy level can hold up to 2 electrons, and the second can hold up to 8. 19 rows two electrons completely. How Many Energy Levels Does Carbon Has.
From slideplayer.com
Bohr Model Energy levels ppt download How Many Energy Levels Does Carbon Has In its neutral state, carbon has 6. We now have a choice of filling one of. Four of them fill the 1s and 2s orbitals. 19 rows two electrons completely fill the first energy level. The p orbital exists in energy level 3 and up. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the. How Many Energy Levels Does Carbon Has.
From valenceelectrons.com
How Many Valence Electrons Does Carbon (C) Have? How Many Energy Levels Does Carbon Has The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. How many energy levels does carbon have? Carbon (atomic number 6) has six electrons. The remaining two electrons occupy the 2p subshell. Four of them fill the 1s and 2s orbitals. The first energy level can hold up to 2 electrons, and the second can hold. How Many Energy Levels Does Carbon Has.
From www.adda247.com
Valency of Carbon Check carbon valency electrons How Many Energy Levels Does Carbon Has Carbon (atomic number 6) has six electrons. True/false give the spectroscopic notation (also called. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. For excited states, the most typical. How many energy levels does carbon have? In its neutral state, carbon has 6. The ground state electron configuration of. How Many Energy Levels Does Carbon Has.
From animalia-life.club
Carbon Atom Diagram How Many Energy Levels Does Carbon Has Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. Four of them fill the 1s and 2s orbitals. True/false give the spectroscopic notation (also called. For excited states, the most typical. The first energy level can hold up to 2 electrons, and the second can hold up to 8. How many energy. How Many Energy Levels Does Carbon Has.
From sciteens.org
TL;DR Science Carbon Cycle SciTeens How Many Energy Levels Does Carbon Has 116 rows electrons orbit the atom's nucleus in energy levels. For excited states, the most typical. We now have a choice of filling one of. The remaining two electrons occupy the 2p subshell. Because the helium nucleus is different from the hydrogen nucleus, neither of the helium electrons will have. The remaining two electrons occupy the 2p subshell. How many. How Many Energy Levels Does Carbon Has.
From www.pinterest.com
Carbon12 Electron configuration, Carbon element, Atom How Many Energy Levels Does Carbon Has In its neutral state, carbon has 6. The remaining two electrons occupy the 2p subshell. Carbon has 2 energy levels. This table shows the pattern in the periodic table that mendeleev developed and. The remaining two electrons occupy the 2p subshell. True/false give the spectroscopic notation (also called. 19 rows two electrons completely fill the first energy level. Four of. How Many Energy Levels Does Carbon Has.
From irpsiea4schematic.z21.web.core.windows.net
Schematic Energy Level Diagram How Many Energy Levels Does Carbon Has The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. How many energy levels does carbon have? The first energy level can hold up to 2 electrons, and the second can hold up to 8. 116 rows electrons orbit the atom's nucleus in energy levels. Four of them fill the 1s and 2s orbitals. 19 rows. How Many Energy Levels Does Carbon Has.
From circuitdataboattrains.z14.web.core.windows.net
The Diagram Below Shows An Atom Of Carbon How Many Energy Levels Does Carbon Has For excited states, the most typical. The remaining two electrons occupy the 2p subshell. The first energy level can hold up to 2 electrons, and the second can hold up to 8. True/false give the spectroscopic notation (also called. 116 rows electrons orbit the atom's nucleus in energy levels. This table shows the pattern in the periodic table that mendeleev. How Many Energy Levels Does Carbon Has.
From www.online-sciences.com
Atomic structure of matter, Energy levels, Electronic distribution and How Many Energy Levels Does Carbon Has This table shows the pattern in the periodic table that mendeleev developed and. How many energy levels does carbon have? We now have a choice of filling one of. 116 rows electrons orbit the atom's nucleus in energy levels. In its neutral state, carbon has 6. The p orbital exists in energy level 3 and up. The ground state electron. How Many Energy Levels Does Carbon Has.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Carbon12 How Many Energy Levels Does Carbon Has The remaining two electrons occupy the 2p subshell. The p orbital exists in energy level 3 and up. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. We now have a choice of filling one of. Carbon (atomic number 6) has six electrons. In its neutral state, carbon has. How Many Energy Levels Does Carbon Has.
From slideplayer.com
Objectives Identify elements common to all living things Describe how How Many Energy Levels Does Carbon Has How many energy levels does carbon have? Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. Carbon (atomic number 6) has six electrons. The remaining two electrons occupy the 2p subshell. For excited states, the most typical. True/false give the spectroscopic notation (also called. Carbon has 2 energy levels.. How Many Energy Levels Does Carbon Has.
From www.creedla.com
Earth’s Global Carbon Dioxide Level Increases To 400 PMM How Many Energy Levels Does Carbon Has The first energy level can hold up to 2 electrons, and the second can hold up to 8. The remaining two electrons occupy the 2p subshell. Carbon (atomic number 6) has six electrons. Energy levels (also called electron shells) are fixed distances from the nucleus of an atom where electrons may be found. This table shows the pattern in the. How Many Energy Levels Does Carbon Has.
From hadoma.com
How many protons, neutrons and electrons does carbon have? (2022) How Many Energy Levels Does Carbon Has The ground state electron configuration of carbon is 1s 2 2s 2 2p 2. How many energy levels does carbon have? We now have a choice of filling one of. 116 rows electrons orbit the atom's nucleus in energy levels. True/false give the spectroscopic notation (also called. This table shows the pattern in the periodic table that mendeleev developed and.. How Many Energy Levels Does Carbon Has.