Lab Titration Assignment . A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Study with quizlet and memorise flashcards containing terms like titrant,. Develop lab skills in operating burets and performing volumetric analysis; Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Calculate the ph of a solution at a specific point during a titration;
from www.studocu.com
Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Study with quizlet and memorise flashcards containing terms like titrant,. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Develop lab skills in operating burets and performing volumetric analysis; Calculate the ph of a solution at a specific point during a titration; A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution.
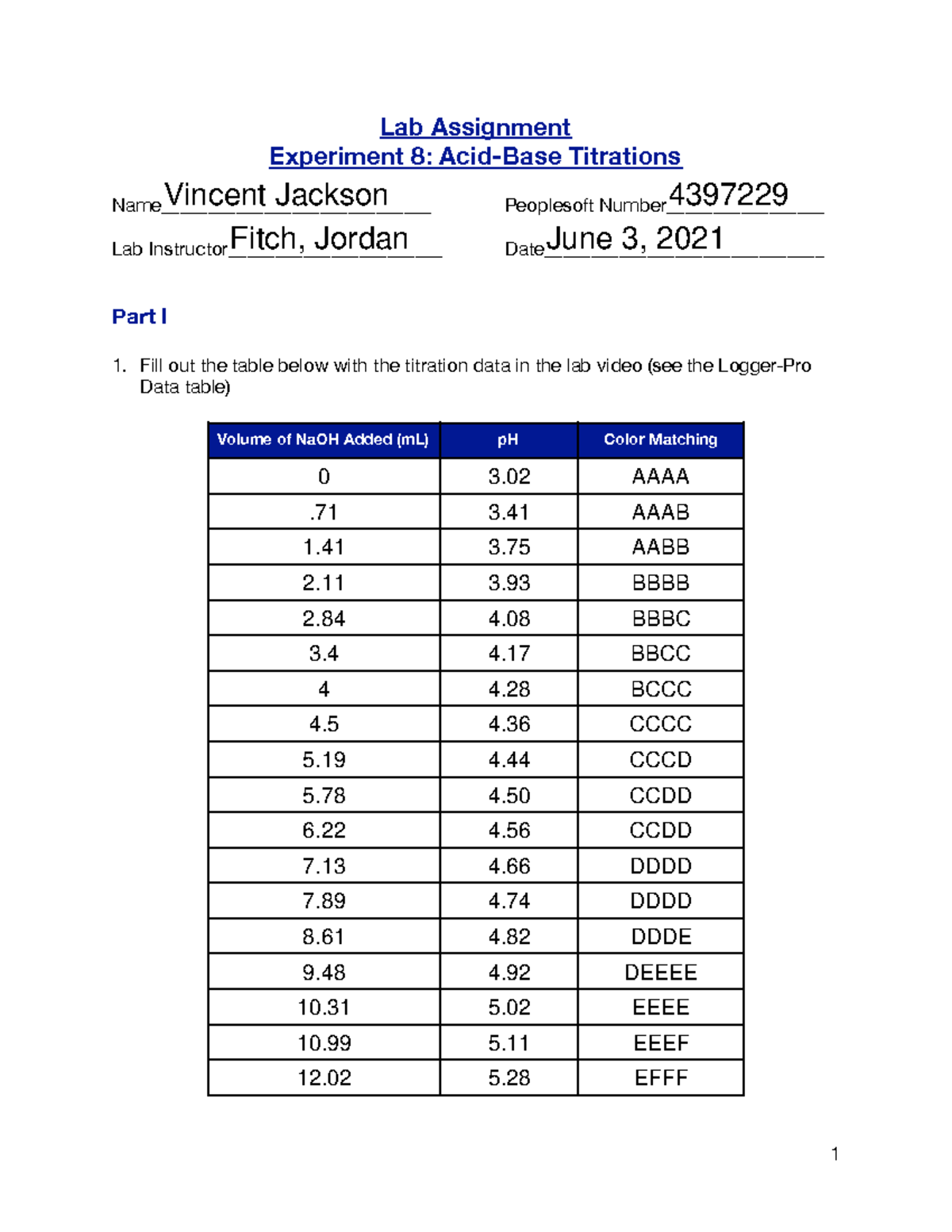
Lab6 feaf Lab Assignment Experiment 8 AcidBase Titrations Name
Lab Titration Assignment A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Develop lab skills in operating burets and performing volumetric analysis; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Calculate the ph of a solution at a specific point during a titration; Study with quizlet and memorise flashcards containing terms like titrant,.
From www.studocu.com
SCH3U Titration Assignment 2021 Titration Simulation Lab Activity Go Lab Titration Assignment Study with quizlet and memorise flashcards containing terms like titrant,. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Calculate the ph of a solution at a specific point during a titration; Develop lab skills in operating burets and performing volumetric analysis; A titration is an analytical. Lab Titration Assignment.
From www.sliderbase.com
Titration Lab Titration Assignment Develop lab skills in operating burets and performing volumetric analysis; In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Be able to determine the k a or k b from ph data. Lab Titration Assignment.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Lab Titration Assignment Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Study with quizlet and memorise flashcards containing terms like titrant,. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titration is the process of determining the concentration of. Lab Titration Assignment.
From mungfali.com
Titration Lab Diagram Lab Titration Assignment Calculate the ph of a solution at a specific point during a titration; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Develop lab skills in operating burets and. Lab Titration Assignment.
From studylib.net
Titration Lab ALTERNATE ASSIGNMENT Lab Titration Assignment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Calculate the. Lab Titration Assignment.
From oneclass.com
OneClass Conductimetric Titration questions. PreLab Assignment Lab Titration Assignment Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Study with quizlet and memorise flashcards containing terms like titrant,. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this experiment you will be determining the volume of. Lab Titration Assignment.
From www.studocu.com
Titration Assignment Module 3 PostLab Questions Submission Module Lab Titration Assignment A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration. Lab Titration Assignment.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Lab Titration Assignment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Calculate the ph of a solution at a specific point during a titration; Be able to determine the k a. Lab Titration Assignment.
From www.studocu.com
Exp.4 Lab Report 824083943 CHEM201 10/28/ Titration Curves Procedure Lab Titration Assignment Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Develop lab skills in operating burets and performing volumetric analysis; Be able to determine the k a or k b. Lab Titration Assignment.
From www.studypool.com
SOLUTION Conductometric titration II lab report Studypool Lab Titration Assignment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Study with quizlet and memorise flashcards containing terms like titrant,. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b. Lab Titration Assignment.
From www.studocu.com
Lab6 feaf Lab Assignment Experiment 8 AcidBase Titrations Name Lab Titration Assignment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Study with quizlet and memorise flashcards containing terms like titrant,. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b. Lab Titration Assignment.
From www.chegg.com
Solved Lab 6 Titrations PostLab Assignment Each student Lab Titration Assignment Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Study with quizlet and memorise flashcards containing terms like titrant,. Develop lab skills in operating burets and performing volumetric analysis;. Lab Titration Assignment.
From joiyfxbtq.blob.core.windows.net
Titration Method Image at Jodie Massey blog Lab Titration Assignment A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Calculate the ph of a solution at a specific point during a titration; Calculate the volume of base for each. Lab Titration Assignment.
From www.studocu.com
Titration Lab Titration Lab Post Lab Assignment Table 1 Vinegar Lab Titration Assignment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Develop lab skills in operating burets and performing volumetric analysis; A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Calculate the volume of base for each trial, average volume. Lab Titration Assignment.
From exyobmsds.blob.core.windows.net
AcidBase Titration Lab Advanced Chemistry With Vernier Answers at Lab Titration Assignment Calculate the ph of a solution at a specific point during a titration; In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Study with quizlet and memorise flashcards containing terms like titrant,. Be. Lab Titration Assignment.
From www.studocu.com
Titration Lab Sheet 1 lab Titration Lab Sheet Sarah Carrasquillo Lab Titration Assignment Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by. Lab Titration Assignment.
From celhcddi.blob.core.windows.net
Titration Lab Math at Mike Brown blog Lab Titration Assignment Calculate the ph of a solution at a specific point during a titration; Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Develop lab skills in operating burets and performing volumetric analysis; Titration is the process of determining the concentration of an unknown solution using a solution of known concentration.. Lab Titration Assignment.
From studylib.net
titrationlabalternativeassignment Lab Titration Assignment A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Calculate the volume of base for each trial, average volume and concentration of the acid of. Lab Titration Assignment.
From www.chegg.com
Solved PreLab Assignment for Experiment 19Titration Curves Lab Titration Assignment Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Develop lab skills in operating burets and performing volumetric analysis; Calculate the ph of a solution at a specific point during a titration; In this experiment you will be determining the volume of sodium hydroxide solution of known. Lab Titration Assignment.
From www.chegg.com
Solved PreLaboratory Assignment AcidBase Titrations Name Lab Titration Assignment A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b from ph data associated with the. Lab Titration Assignment.
From www.studocu.com
WEEK 9 LAB Activity Titration Week 9 Unit Task Titration Illustrate Lab Titration Assignment Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration. Lab Titration Assignment.
From joizlgxlm.blob.core.windows.net
Titration Experiments In Supramolecular Chemistry at Larry Ferrill blog Lab Titration Assignment Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Develop lab skills in operating burets and performing volumetric analysis; A titration is an analytical procedure. Lab Titration Assignment.
From www.studocu.com
Lab Titration edgenuity assignment Title Purpose Explore the Lab Titration Assignment Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b from ph data associated with the titration of a weak. Lab Titration Assignment.
From www.studocu.com
Lab Report For Physics Edgenuity Online Solubility Lab Report Lab Titration Assignment Study with quizlet and memorise flashcards containing terms like titrant,. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In this experiment you will be determining the volume of. Lab Titration Assignment.
From studylib.net
Acid/Base Titration Lab Lab Titration Assignment Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Study with quizlet and memorise flashcards containing terms like titrant,. A titration is an analytical procedure used to determine the accurate concentration of a. Lab Titration Assignment.
From klaqnshvp.blob.core.windows.net
How To Do A Titration Rsc at Paul Gallegos blog Lab Titration Assignment Study with quizlet and memorise flashcards containing terms like titrant,. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. In this experiment you will be. Lab Titration Assignment.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Lab Titration Assignment Study with quizlet and memorise flashcards containing terms like titrant,. Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titration is the process of determining the concentration of. Lab Titration Assignment.
From studylib.net
Acid Base Titration Lab Lab Titration Assignment Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A. Lab Titration Assignment.
From www.studocu.com
Week 11 Titration Prelab Summary PreLab Assignment Titration (Week Lab Titration Assignment Study with quizlet and memorise flashcards containing terms like titrant,. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Calculate the ph of a solution at a specific point during a titration; A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with. Lab Titration Assignment.
From betterlesson.com
Eleventh grade Lesson Titration Lab BetterLesson Lab Titration Assignment In this experiment you will be determining the volume of sodium hydroxide solution of known concentration required to. Develop lab skills in operating burets and performing volumetric analysis; Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. A titration is an analytical procedure used to determine the. Lab Titration Assignment.
From mavink.com
Acid Base Titration Experiment Lab Titration Assignment Calculate the ph of a solution at a specific point during a titration; Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. A titration is an analytical procedure used. Lab Titration Assignment.
From www.studocu.com
Titration Report BTEC Applied Science Unit2 A Titration Report Lab Titration Assignment Be able to determine the k a or k b from ph data associated with the titration of a weak acid or. Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Calculate the ph of a solution at a specific point during a titration; A titration is an analytical procedure. Lab Titration Assignment.
From www.studocu.com
Pre PreLab Assignment Titrations PreLab Assignment Titrations Lab Titration Assignment Calculate the volume of base for each trial, average volume and concentration of the acid of a titration where. Calculate the ph of a solution at a specific point during a titration; A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. In this experiment you will be. Lab Titration Assignment.
From www.chegg.com
Solved LAB PREP ASSIGNMENT 09 REDOX TITRATIONS The lab prep Lab Titration Assignment Develop lab skills in operating burets and performing volumetric analysis; A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Titration is the process of determining the concentration of an unknown solution using a solution of known concentration. Calculate the ph of a solution at a specific point. Lab Titration Assignment.
From listens.online
pre lab assignment titration of vinegar Lab Titration Assignment A titration is an analytical procedure used to determine the accurate concentration of a sample by reacting it with a standard solution. Study with quizlet and memorise flashcards containing terms like titrant,. Develop lab skills in operating burets and performing volumetric analysis; Be able to determine the k a or k b from ph data associated with the titration of. Lab Titration Assignment.