Which Of The Following Statements About The Molecule O2 Is False . He2 molecule does not exist as the effect of bonding and anti. Which of the following statements about the molecule o2 is false?a. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. Which of the following statements regarding the oxygen atom of a water molecule is true? O2 has one sigma bond and one pi bond. Its bond order is 2.b. The number of valence electrons is 12.c. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. The false statement about the molecule o2 is that it has two pi bonds. Ozone is an allotrope of. The most common form of oxygen is a diatomic molecule. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. A) oxygen is more positively charged than the. Which of the following statements about oxygen is false?
from www.bartleby.com
Which of the following statements about oxygen is false? Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. The number of valence electrons is 12.c. The most common form of oxygen is a diatomic molecule. The false statement about the molecule o2 is that it has two pi bonds. A) oxygen is more positively charged than the. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. Ozone is an allotrope of. Which of the following statements about the molecule o2 is false?a. Its bond order is 2.b.
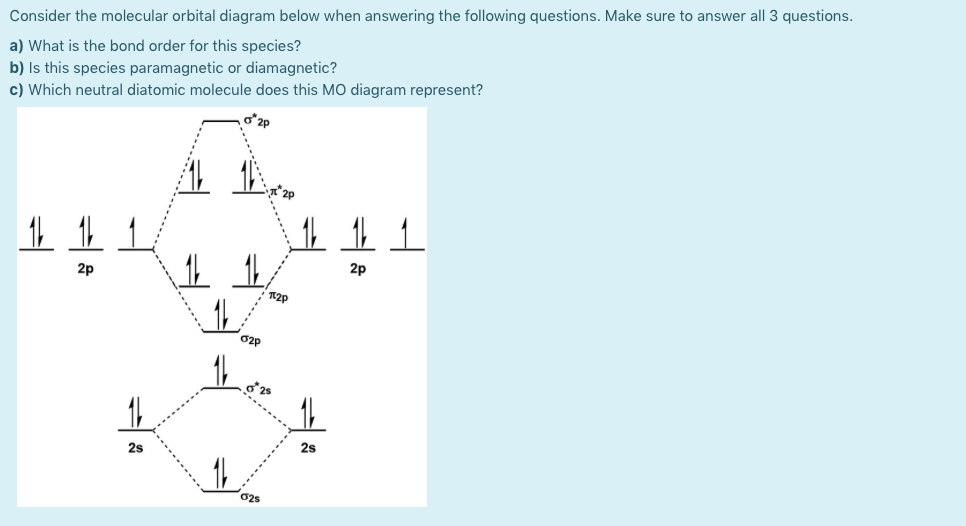
Answered Consider the molecular orbital diagram… bartleby
Which Of The Following Statements About The Molecule O2 Is False Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. Which of the following statements about the molecule o2 is false?a. Ozone is an allotrope of. Its bond order is 2.b. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. Which of the following statements regarding the oxygen atom of a water molecule is true? The false statement about the molecule o2 is that it has two pi bonds. The most common form of oxygen is a diatomic molecule. Which of the following statements about oxygen is false? O2 has one sigma bond and one pi bond. A) oxygen is more positively charged than the. He2 molecule does not exist as the effect of bonding and anti. The number of valence electrons is 12.c.
From www.chegg.com
Solved Which of the following statements concerning Which Of The Following Statements About The Molecule O2 Is False A) oxygen is more positively charged than the. He2 molecule does not exist as the effect of bonding and anti. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. Which of the following statements regarding the oxygen atom of a water molecule is true? Co is a polar molecule and has a dipole moment, while o2. Which Of The Following Statements About The Molecule O2 Is False.
From www.numerade.com
SOLVED Which of the following statements are false about chemical Which Of The Following Statements About The Molecule O2 Is False Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. Which of the following statements about oxygen is false? Which of the following statements about the molecule o2 is false?a. He2 molecule does not exist as the effect of bonding and anti. Its bond order is 2.b. The number of valence electrons is 12.c. Ozone is an. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved In the catalyzed reaction an iodine radical attacks Which Of The Following Statements About The Molecule O2 Is False Which of the following statements regarding the oxygen atom of a water molecule is true? The most common form of oxygen is a diatomic molecule. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. O2 has one sigma bond and one pi bond. The number of valence electrons is 12.c. Co is a polar molecule and. Which Of The Following Statements About The Molecule O2 Is False.
From www.bartleby.com
Answered nthetic organisms release only O2… bartleby Which Of The Following Statements About The Molecule O2 Is False Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. The most common form of oxygen is a diatomic molecule. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. A) oxygen is more positively charged than the. Study with quizlet and memorize flashcards. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved 1. Which of the following statements concerning Which Of The Following Statements About The Molecule O2 Is False Which of the following statements regarding the oxygen atom of a water molecule is true? Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. The number of valence electrons is 12.c. The most common form of oxygen is a diatomic molecule. The false statement about the molecule o2 is that it has two pi bonds. He2. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Question 22 Which of the following statements Which Of The Following Statements About The Molecule O2 Is False Which of the following statements about oxygen is false? A) oxygen is more positively charged than the. Which of the following statements regarding the oxygen atom of a water molecule is true? Ozone is an allotrope of. Which of the following statements about the molecule o2 is false?a. Co is a polar molecule and has a dipole moment, while o2. Which Of The Following Statements About The Molecule O2 Is False.
From www.researchgate.net
Molecular oxygen (O 2 ) as a biosignature, false positives, and Which Of The Following Statements About The Molecule O2 Is False Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. Which of the following statements regarding the oxygen atom of a water molecule is true? O2 has one sigma bond and one pi bond. Its bond order is 2.b. A) oxygen is more positively charged than the. The number of valence electrons is 12.c. The most common. Which Of The Following Statements About The Molecule O2 Is False.
From www.numerade.com
SOLVED Are the following statements true or false? For monoatomic Which Of The Following Statements About The Molecule O2 Is False The most common form of oxygen is a diatomic molecule. Which of the following statements about oxygen is false? Its bond order is 2.b. Ozone is an allotrope of. Which of the following statements about the molecule o2 is false?a. Which of the following statements regarding the oxygen atom of a water molecule is true? O2 has one sigma bond. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved I. Indicate whether the following statements are true Which Of The Following Statements About The Molecule O2 Is False Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. O2 has one sigma bond and one pi bond. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. The false statement about the molecule o2 is that it has two pi bonds. Which of the following. Which Of The Following Statements About The Molecule O2 Is False.
From www.pinterest.co.kr
Covalent bonding in an oxygen molecule. Covalent bonding, Chemistry Which Of The Following Statements About The Molecule O2 Is False O2 has one sigma bond and one pi bond. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. He2 molecule does not exist as the effect of bonding and anti. The most common form of oxygen is a diatomic molecule. Its bond order is 2.b. Which of the following. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved For the following statements answer true or false Which Of The Following Statements About The Molecule O2 Is False Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. The number of valence electrons is 12.c. The most common form of oxygen is a diatomic molecule. He2 molecule does not exist as the effect of bonding and anti. A) oxygen is more positively charged than the. Ozone is an. Which Of The Following Statements About The Molecule O2 Is False.
From brainly.com
What is the name of the molecule shown below? Which Of The Following Statements About The Molecule O2 Is False Its bond order is 2.b. Which of the following statements regarding the oxygen atom of a water molecule is true? Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. Which of the following statements about the molecule o2 is false?a. Co is a polar molecule and has a dipole. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Part A Which of the following statements about water Which Of The Following Statements About The Molecule O2 Is False The false statement about the molecule o2 is that it has two pi bonds. O2 has one sigma bond and one pi bond. Which of the following statements about the molecule o2 is false?a. A) oxygen is more positively charged than the. Ozone is an allotrope of. Co is a polar molecule and has a dipole moment, while o2 is. Which Of The Following Statements About The Molecule O2 Is False.
From schematicpiscardixf.z4.web.core.windows.net
O2 Bond Order Diagram Which Of The Following Statements About The Molecule O2 Is False Ozone is an allotrope of. The false statement about the molecule o2 is that it has two pi bonds. Its bond order is 2.b. Which of the following statements about oxygen is false? Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. Which of the following statements about the molecule o2 is false?a. Which of the. Which Of The Following Statements About The Molecule O2 Is False.
From www.numerade.com
SOLVED Consider a molecule of PBr3 and determine if each of the Which Of The Following Statements About The Molecule O2 Is False Its bond order is 2.b. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. O2 has one sigma bond and one pi bond. A) oxygen is more positively charged than the. Which of the following statements regarding the oxygen atom of a water molecule is true? Which of the. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Use the molecular orbital model to fully describe the Which Of The Following Statements About The Molecule O2 Is False Which of the following statements about oxygen is false? Which of the following statements about the molecule o2 is false?a. Its bond order is 2.b. The number of valence electrons is 12.c. He2 molecule does not exist as the effect of bonding and anti. The false statement about the molecule o2 is that it has two pi bonds. Which of. Which Of The Following Statements About The Molecule O2 Is False.
From www.bartleby.com
Answered Consider the molecular orbital diagram… bartleby Which Of The Following Statements About The Molecule O2 Is False Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. The most common form of oxygen is a diatomic molecule. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. Ozone is an allotrope of. A) oxygen is more positively charged than the. The. Which Of The Following Statements About The Molecule O2 Is False.
From guidedehartsicklewort.z21.web.core.windows.net
Molecular Orbital Diagram Of O2 Molecule Which Of The Following Statements About The Molecule O2 Is False O2 has one sigma bond and one pi bond. Which of the following statements regarding the oxygen atom of a water molecule is true? Which of the following statements about oxygen is false? A) oxygen is more positively charged than the. The most common form of oxygen is a diatomic molecule. Which of the following statements about the molecule o2. Which Of The Following Statements About The Molecule O2 Is False.
From gadgetssai.com
Which Of The Following Statements About Water Is False GadgetsSai Which Of The Following Statements About The Molecule O2 Is False Its bond order is 2.b. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. Ozone is an allotrope of. O2 has one sigma bond and one pi bond. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Molecular Orbital Theory Homodiatom Use The Molecu... Which Of The Following Statements About The Molecule O2 Is False He2 molecule does not exist as the effect of bonding and anti. Which of the following statements regarding the oxygen atom of a water molecule is true? The false statement about the molecule o2 is that it has two pi bonds. O2 has one sigma bond and one pi bond. Among o+ 2,o2 and o− 2 the stability decreases as. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Use The Molecular Orbital Diagram Below To Determi... Which Of The Following Statements About The Molecule O2 Is False Which of the following statements about the molecule o2 is false?a. A) oxygen is more positively charged than the. The most common form of oxygen is a diatomic molecule. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. He2 molecule does not exist as the effect of bonding and. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Question 8 True/False Statements in DNA Replication Which Of The Following Statements About The Molecule O2 Is False Which of the following statements about the molecule o2 is false?a. O2 has one sigma bond and one pi bond. Ozone is an allotrope of. The number of valence electrons is 12.c. Which of the following statements about oxygen is false? A) oxygen is more positively charged than the. Which of the following statements regarding the oxygen atom of a. Which Of The Following Statements About The Molecule O2 Is False.
From courses.lumenlearning.com
Chemical Bonds BIO103 Human Biology Which Of The Following Statements About The Molecule O2 Is False Its bond order is 2.b. The number of valence electrons is 12.c. Study with quizlet and memorize flashcards containing terms like which of the following statements regarding the oxygen atom of a. O2 has one sigma bond and one pi bond. The most common form of oxygen is a diatomic molecule. Which of the following statements about the molecule o2. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Question 1 Which of the following statements are Which Of The Following Statements About The Molecule O2 Is False Ozone is an allotrope of. The most common form of oxygen is a diatomic molecule. Its bond order is 2.b. The number of valence electrons is 12.c. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. O2 has one sigma bond and one pi bond. Among. Which Of The Following Statements About The Molecule O2 Is False.
From www.numerade.com
SOLVED Given a molecular orbital diagram below; determine if each of Which Of The Following Statements About The Molecule O2 Is False Ozone is an allotrope of. The most common form of oxygen is a diatomic molecule. He2 molecule does not exist as the effect of bonding and anti. Which of the following statements about the molecule o2 is false?a. O2 has one sigma bond and one pi bond. The number of valence electrons is 12.c. Among o+ 2,o2 and o− 2. Which Of The Following Statements About The Molecule O2 Is False.
From www.numerade.com
SOLVED Question 3 (6.06 points) Which one of the statements about the Which Of The Following Statements About The Molecule O2 Is False Ozone is an allotrope of. He2 molecule does not exist as the effect of bonding and anti. Its bond order is 2.b. Which of the following statements about oxygen is false? A) oxygen is more positively charged than the. The most common form of oxygen is a diatomic molecule. The number of valence electrons is 12.c. Co is a polar. Which Of The Following Statements About The Molecule O2 Is False.
From proper-cooking.info
Oxygen Molecular Diagram Which Of The Following Statements About The Molecule O2 Is False Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. Its bond order is 2.b. O2 has one sigma bond and one pi bond. A) oxygen is more positively charged than the. The false statement about the molecule o2 is that it has two pi bonds. The. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Determine whether the following statements are true Which Of The Following Statements About The Molecule O2 Is False He2 molecule does not exist as the effect of bonding and anti. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. O2 has one sigma bond and one pi bond. Ozone is an allotrope of. Among o+ 2,o2 and o− 2 the stability decreases as o+. Which Of The Following Statements About The Molecule O2 Is False.
From studylib.net
ZumdahlSampleCh Which Of The Following Statements About The Molecule O2 Is False He2 molecule does not exist as the effect of bonding and anti. The number of valence electrons is 12.c. Its bond order is 2.b. Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. The most common form of oxygen is a diatomic molecule. Ozone is an allotrope of. Co is a polar molecule and has a. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Which of the following is/are true about the Which Of The Following Statements About The Molecule O2 Is False O2 has one sigma bond and one pi bond. Which of the following statements regarding the oxygen atom of a water molecule is true? Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. He2 molecule does not exist as the effect of bonding and anti. A) oxygen is more positively charged than the. Ozone is an. Which Of The Following Statements About The Molecule O2 Is False.
From kunduz.com
[ANSWERED] The IUPAC name for the following molecule is i i HO O2 4 8 Which Of The Following Statements About The Molecule O2 Is False Which of the following statements regarding the oxygen atom of a water molecule is true? Among o+ 2,o2 and o− 2 the stability decreases as o+ 2>o2>o− 2. Ozone is an allotrope of. The number of valence electrons is 12.c. He2 molecule does not exist as the effect of bonding and anti. O2 has one sigma bond and one pi. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved complete each of the following statements for a Which Of The Following Statements About The Molecule O2 Is False Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. He2 molecule does not exist as the effect of bonding and anti. The number of valence electrons is 12.c. The false statement about the molecule o2 is that it has two pi bonds. Its bond order is. Which Of The Following Statements About The Molecule O2 Is False.
From manuallistgranitise.z1.web.core.windows.net
Molecular Orbital Diagram For O2 Which Of The Following Statements About The Molecule O2 Is False Which of the following statements about oxygen is false? The most common form of oxygen is a diatomic molecule. A) oxygen is more positively charged than the. Ozone is an allotrope of. The false statement about the molecule o2 is that it has two pi bonds. Study with quizlet and memorize flashcards containing terms like which of the following statements. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Label the following diagram of water molecules, Which Of The Following Statements About The Molecule O2 Is False He2 molecule does not exist as the effect of bonding and anti. Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. The number of valence electrons is 12.c. Which of the following statements about oxygen is false? The false statement about the molecule o2 is that. Which Of The Following Statements About The Molecule O2 Is False.
From www.chegg.com
Solved Which of the following statements regarding enhancers Which Of The Following Statements About The Molecule O2 Is False Co is a polar molecule and has a dipole moment, while o2 is a nonpolar molecule and does not have a dipole moment. The number of valence electrons is 12.c. O2 has one sigma bond and one pi bond. Ozone is an allotrope of. The false statement about the molecule o2 is that it has two pi bonds. Study with. Which Of The Following Statements About The Molecule O2 Is False.