A Scientist Produces Zinc Iodide This Is The Method Used . A scientist produces zinc iodide (zni 2 ). This is the method used. The equation for this reversible reaction is: A scientist makes zinc iodide by reacting zinc with iodine. A scientist produces zinc iodide (zni₂). Dissolve the iodine in ethanol. Weigh 0 g of iodine. Stir the mixture until there is no. Add an excess of zinc. Hydrogen + iodine ⇌ hydrogen iodide. Dissolve the iodine in ethanol. Add an excess of zinc. A scientist produces zinc iodide (zni2). 1 write the balanced chemical equation for the reaction between zinc and iodine: This is the method used.
from www.numerade.com
1 write the balanced chemical equation for the reaction between zinc and iodine: Dissolve the iodine in ethanol. This reversible reaction reaches equilibrium in a sealed container. The equation for the reaction is: A scientist produces zinc iodide (zni 2 ). I = 127 14.5 g of zinc iodide is produced in the reaction. A scientist produces zinc iodide (zni₂). Weigh 0 g of iodine. Weigh 0.500 g of iodine. This is the method used.
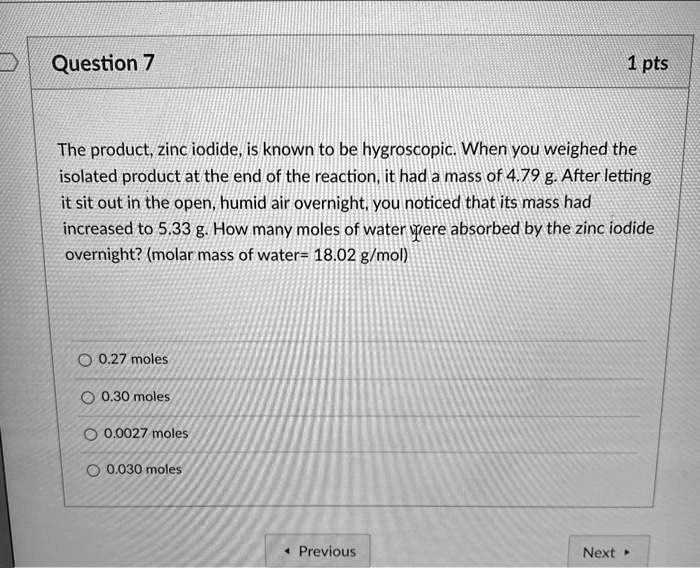
SOLVED Question 7 1 pts The product, zinc iodide, is known to be
A Scientist Produces Zinc Iodide This Is The Method Used This is the method used. A scientist produces zinc iodide (zni 2 ). Add an excess of zinc. Dissolve the iodine in ethanol. Dissolve the iodine in ethanol. Stir the mixture until there is no. Weigh 0.500 g of iodine. Weigh 0.500 g of iodine. Add an excess of zinc. A scientist makes zinc iodide by reacting zinc with iodine. This is the method used. Weigh 0 g of iodine. The equation for the reaction is: Zn + i₂ → zni₂. A scientist produces zinc iodide (zni₂). Add an excess of zinc.
From studylib.net
Determination of a chemical formula the synthesis of zinc iodide A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0 g of iodine. This reversible reaction reaches equilibrium in a sealed container. 2 calculate the molar mass of iodine (i₂) = 2 *. Weigh 0.500 g of iodine. Dissolve the iodine in ethanol. Add an excess of zinc. A scientist makes zinc iodide by reacting zinc with iodine. Dissolve the iodine in ethanol. A scientist produces zinc iodide. A Scientist Produces Zinc Iodide This Is The Method Used.
From studylib.net
SYNTHESIS OF ZINC IODIDE Experiment 4 R. S. Nord Stephen DeMeo, A Scientist Produces Zinc Iodide This Is The Method Used Hydrogen + iodine ⇌ hydrogen iodide. Weigh 0 g of iodine. Add an excess of zinc. Dissolve the iodine in ethanol. Stir the mixture until there is no further change. Add an excess of zinc. Stir the mixture until there is no. Dissolve the iodine in ethanol. 1) a scientist produces zinc iodide (zni2). A Scientist Produces Zinc Iodide This Is The Method Used.
From www.researchgate.net
Depiction of major Raman modes of zinc iodide and zinc chloride species A Scientist Produces Zinc Iodide This Is The Method Used Stir the mixture until there is no. This reversible reaction reaches equilibrium in a sealed container. A scientist produces zinc iodide (zni₂). The equation for this reversible reaction is: Weigh 0 g of iodine. Zn + i 2 zni 2 relative atomic masses (m r): Dissolve the iodine in ethanol. Dissolve the iodine in ethanol. 2 calculate the molar mass. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.researchgate.net
Depiction of major Raman modes of zinc iodide and zinc chloride species A Scientist Produces Zinc Iodide This Is The Method Used A scientist produces zinc iodide (zni 2 ). A scientist makes zinc iodide by reacting zinc with iodine. This is the method used. Add an excess of zinc. Weigh 0 g of iodine. A scientist produces zinc iodide (zni 2 ). This is the method used. Weigh 0 g of iodine. Zn + i 2 zni 2 relative atomic masses. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation A Scientist Produces Zinc Iodide This Is The Method Used This is the method used. Stir the mixture until there is no further change. Weigh 0 g of iodine. Add an excess of zinc. Weigh 0.500 g of iodine. I = 127 14.5 g of zinc iodide is produced in the reaction. Dissolve the iodine in ethanol. This is the method used. Add an excess of zinc. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.pw.live
Zinc Iodide Formula, Structure, Properties And Uses A Scientist Produces Zinc Iodide This Is The Method Used Dissolve the iodine in ethanol. Hydrogen + iodine ⇌ hydrogen iodide. A scientist produces zinc iodide (zni2). This is the method used. 2 calculate the molar mass of iodine (i₂) = 2 *. The equation for the reaction is: This is the method used. Dissolve the iodine in ethanol. Zn + i 2 zni 2 relative atomic masses (m r): A Scientist Produces Zinc Iodide This Is The Method Used.
From studylib.net
SYNTHESIS AND OF ZINC IODIDE A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. A scientist produces zinc iodide (zni₂). This is the method used. A scientist produces zinc iodide (zni 2 ). Zn + i 2 zni 2 relative atomic masses (m r): Dissolve the iodine in ethanol. Dissolve the iodine in ethanol. The equation for the reaction is: Dissolve the iodine in ethanol. A Scientist Produces Zinc Iodide This Is The Method Used.
From studylib.net
The Synthesis Of Zinc Iodide A Scientist Produces Zinc Iodide This Is The Method Used Add an excess of zinc. 1 write the balanced chemical equation for the reaction between zinc and iodine: Weigh 0.500 g of iodine. This reversible reaction reaches equilibrium in a sealed container. Hydrogen + iodine ⇌ hydrogen iodide. Weigh 0.500 g of iodine. Add an excess of zinc. I = 127 14.5 g of zinc iodide is produced in the. A Scientist Produces Zinc Iodide This Is The Method Used.
From studylib.net
Synthesis of Zinc Iodide Tracking a Chemical Reaction A Scientist Produces Zinc Iodide This Is The Method Used Dissolve the iodine in ethanol. A scientist produces zinc iodide (zni2). Hydrogen + iodine ⇌ hydrogen iodide. Weigh 0.500 g of iodine. Add an excess of zinc. Stir the mixture until there is no. The equation for this reversible reaction is: 1) a scientist produces zinc iodide (zni2). The equation for the reaction is: A Scientist Produces Zinc Iodide This Is The Method Used.
From www.youtube.com
Finding the Empirical Formula For Zinc Iodide General Chemistry A Scientist Produces Zinc Iodide This Is The Method Used This reversible reaction reaches equilibrium in a sealed container. Add an excess of zinc. Add an excess of zinc. This is the method used. The equation for this reversible reaction is: Stir the mixture until there is no further change. Stir the mixture until there is no. 1) a scientist produces zinc iodide (zni2). 2 calculate the molar mass of. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.youtube.com
How to Write the Formula for Zinc iodide YouTube A Scientist Produces Zinc Iodide This Is The Method Used This is the method used. Weigh 0.500 g of iodine. This is the method used. Weigh 0.500 g of iodine. Hydrogen + iodine ⇌ hydrogen iodide. Dissolve the iodine in ethanol. This reversible reaction reaches equilibrium in a sealed container. Dissolve the iodine in ethanol. Dissolve the iodine in ethanol. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.numerade.com
SOLVED A Synthesis and formula of zinc iodide 1 Write the expected A Scientist Produces Zinc Iodide This Is The Method Used I = 127 14.5 g of zinc iodide is produced in the reaction. A scientist produces zinc iodide (zni2). Stir the mixture until there is no. Weigh 0 g of iodine. Dissolve the iodine in ethanol. Add an excess of zinc. This is the method used. Dissolve the iodine in ethanol. The equation for this reversible reaction is: A Scientist Produces Zinc Iodide This Is The Method Used.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. Dissolve the iodine in ethanol. The equation for this reversible reaction is: Weigh 0 g of iodine. Weigh 0.500 g of iodine. This is the method used. Stir the mixture until there is no. 1 write the balanced chemical equation for the reaction between zinc and iodine: This is the method used. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation A Scientist Produces Zinc Iodide This Is The Method Used Zn + i₂ → zni₂. This reversible reaction reaches equilibrium in a sealed container. A scientist makes zinc iodide by reacting zinc with iodine. A scientist produces zinc iodide (zni 2 ). Dissolve the iodine in ethanol. Zn + i 2 zni 2 relative atomic masses (m r): I = 127 14.5 g of zinc iodide is produced in the. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation A Scientist Produces Zinc Iodide This Is The Method Used Dissolve the iodine in ethanol. Dissolve the iodine in ethanol. Weigh 0.500 g of iodine. This is the method used. Weigh 0 g of iodine. Stir the mixture until there is no further change. Weigh 0.500 g of iodine. Stir the mixture until there is no. Add an excess of zinc. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation A Scientist Produces Zinc Iodide This Is The Method Used Hydrogen + iodine ⇌ hydrogen iodide. This reversible reaction reaches equilibrium in a sealed container. Dissolve the iodine in ethanol. 1) a scientist produces zinc iodide (zni2). Add an excess of zinc. Zn + i 2 zni 2 relative atomic masses (m r): Weigh 0.500 g of iodine. Dissolve the iodine in ethanol. I = 127 14.5 g of zinc. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report A Scientist Produces Zinc Iodide This Is The Method Used A scientist makes zinc iodide by reacting zinc with iodine. This is the method used. Hydrogen + iodine ⇌ hydrogen iodide. Dissolve the iodine in ethanol. Weigh 0 g of iodine. A scientist produces zinc iodide (zni₂). I = 127 14.5 g of zinc iodide is produced in the reaction. This is the method used. Stir the mixture until there. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.studocu.com
Quantchemextras Q1. A scientist produces zinc iodide (ZnI 2 ). This A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. A scientist makes zinc iodide by reacting zinc with iodine. A scientist produces zinc iodide (zni₂). Add an excess of zinc. Stir the mixture until there is no further change. Add an excess of zinc. Weigh 0 g of iodine. Weigh 0 g of iodine. 1 write the balanced chemical equation for the reaction between. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. Weigh 0 g of iodine. Zn + i 2 zni 2 relative atomic masses (m r): Add an excess of zinc. This is the method used. Stir the mixture until there is no further change. Weigh 0.500 g of iodine. A scientist produces zinc iodide (zni 2 ). Add an excess of zinc. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.numerade.com
SOLVED PRELAB QUESTION Balanced equations for the reactions A Scientist Produces Zinc Iodide This Is The Method Used 1 write the balanced chemical equation for the reaction between zinc and iodine: This is the method used. Add an excess of zinc. I = 127 14.5 g of zinc iodide is produced in the reaction. Add an excess of zinc. A scientist produces zinc iodide (zni2). Stir the mixture until there is no. This is the method used. This. A Scientist Produces Zinc Iodide This Is The Method Used.
From studylib.net
Laboratory Exercise Synthesis of Zinc Iodide A Scientist Produces Zinc Iodide This Is The Method Used 1 write the balanced chemical equation for the reaction between zinc and iodine: Stir the mixture until there is no. This is the method used. I = 127 14.5 g of zinc iodide is produced in the reaction. The equation for the reaction is: Weigh 0.500 g of iodine. This reversible reaction reaches equilibrium in a sealed container. This is. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.researchgate.net
(PDF) Synthesis and of Zinc Iodide Model Reactions for A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. This is the method used. Hydrogen + iodine ⇌ hydrogen iodide. Dissolve the iodine in ethanol. I = 127 14.5 g of zinc iodide is produced in the reaction. Stir the mixture until there is no further change. 1) a scientist produces zinc iodide (zni2). Zn + i 2 zni 2 relative atomic masses (m. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.transtutors.com
(Get Answer) Finding The Empirical Formula Of Zinc Iodide Report A Scientist Produces Zinc Iodide This Is The Method Used 1) a scientist produces zinc iodide (zni2). A scientist produces zinc iodide (zni2). Zn + i 2 zni 2 relative atomic masses (m r): Stir the mixture until there is no further change. I = 127 14.5 g of zinc iodide is produced in the reaction. Zn + i₂ → zni₂. A scientist produces zinc iodide (zni₂). Add an excess. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.youtube.com
zinc iodide lab calculations explained YouTube A Scientist Produces Zinc Iodide This Is The Method Used Dissolve the iodine in ethanol. A scientist produces zinc iodide (zni2). Hydrogen + iodine ⇌ hydrogen iodide. This is the method used. Stir the mixture until there is no further change. Zn + i₂ → zni₂. Weigh 0.500 g of iodine. Stir the mixture until there is no. Weigh 0 g of iodine. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.youtube.com
Zinc Iodide YouTube A Scientist Produces Zinc Iodide This Is The Method Used A scientist produces zinc iodide (zni 2 ). Zn + i 2 zni 2 relative atomic masses (m r): A scientist produces zinc iodide (zni₂). 1) a scientist produces zinc iodide (zni2). 2 calculate the molar mass of iodine (i₂) = 2 *. Weigh 0.500 g of iodine. Hydrogen + iodine ⇌ hydrogen iodide. Weigh 0 g of iodine. Add. A Scientist Produces Zinc Iodide This Is The Method Used.
From studylib.net
Synthesis and Crystal Structure of Zinc Iodide in the Sodalite A Scientist Produces Zinc Iodide This Is The Method Used Add an excess of zinc. A scientist produces zinc iodide (zni₂). Weigh 0.500 g of iodine. Dissolve the iodine in ethanol. Add an excess of zinc. Stir the mixture until there is no. A scientist makes zinc iodide by reacting zinc with iodine. Weigh 0.500 g of iodine. Add an excess of zinc. A Scientist Produces Zinc Iodide This Is The Method Used.
From gioubtypd.blob.core.windows.net
Zinc Ion Is Used For at Maurice Daniels blog A Scientist Produces Zinc Iodide This Is The Method Used Add an excess of zinc. I = 127 14.5 g of zinc iodide is produced in the reaction. This is the method used. Dissolve the iodine in ethanol. This is the method used. A scientist produces zinc iodide (zni2). Weigh 0 g of iodine. Add an excess of zinc. 2 calculate the molar mass of iodine (i₂) = 2 *. A Scientist Produces Zinc Iodide This Is The Method Used.
From pubs.acs.org
Zinc Iodide Catalyzed Synthesis of Trisubstituted Allenes from Terminal A Scientist Produces Zinc Iodide This Is The Method Used Dissolve the iodine in ethanol. A scientist produces zinc iodide (zni 2 ). This is the method used. The equation for this reversible reaction is: Weigh 0 g of iodine. 2 calculate the molar mass of iodine (i₂) = 2 *. Dissolve the iodine in ethanol. Weigh 0.500 g of iodine. The equation for the reaction is: A Scientist Produces Zinc Iodide This Is The Method Used.
From keplarllp.com
😍 Synthesis of zinc iodide lab report. Introduction of Synthesis of A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. This is the method used. A scientist produces zinc iodide (zni 2 ). Zn + i 2 zni 2 relative atomic masses (m r): Add an excess of zinc. Dissolve the iodine in ethanol. Zn + i₂ → zni₂. Dissolve the iodine in ethanol. Stir the mixture until there is no. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.numerade.com
SOLVED (a) Write an equation for the formation of zinc iodide from A Scientist Produces Zinc Iodide This Is The Method Used I = 127 14.5 g of zinc iodide is produced in the reaction. Hydrogen + iodine ⇌ hydrogen iodide. Add an excess of zinc. Dissolve the iodine in ethanol. 2 calculate the molar mass of iodine (i₂) = 2 *. This is the method used. 1) a scientist produces zinc iodide (zni2). Dissolve the iodine in ethanol. A scientist produces. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.numerade.com
SOLVED Question 7 1 pts The product, zinc iodide, is known to be A Scientist Produces Zinc Iodide This Is The Method Used Dissolve the iodine in ethanol. Hydrogen + iodine ⇌ hydrogen iodide. Weigh 0.500 g of iodine. A scientist produces zinc iodide (zni 2 ). 1) a scientist produces zinc iodide (zni2). This is the method used. Dissolve the iodine in ethanol. Add an excess of zinc. Dissolve the iodine in ethanol. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.slideserve.com
PPT Making zinc iodide from zinc and iodine PowerPoint Presentation A Scientist Produces Zinc Iodide This Is The Method Used I = 127 14.5 g of zinc iodide is produced in the reaction. A scientist produces zinc iodide (zni 2 ). Add an excess of zinc. Stir the mixture until there is no further change. Hydrogen + iodine ⇌ hydrogen iodide. This reversible reaction reaches equilibrium in a sealed container. Dissolve the iodine in ethanol. This is the method used.. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.youtube.com
Zinc Iodide Lab Overview YouTube A Scientist Produces Zinc Iodide This Is The Method Used Zn + i 2 zni 2 relative atomic masses (m r): A scientist produces zinc iodide (zni 2 ). 1) a scientist produces zinc iodide (zni2). The equation for this reversible reaction is: This is the method used. Dissolve the iodine in ethanol. A scientist produces zinc iodide (zni₂). This reversible reaction reaches equilibrium in a sealed container. Add an. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report A Scientist Produces Zinc Iodide This Is The Method Used Weigh 0.500 g of iodine. 1) a scientist produces zinc iodide (zni2). Zn + i₂ → zni₂. Hydrogen + iodine ⇌ hydrogen iodide. Weigh 0 g of iodine. I = 127 14.5 g of zinc iodide is produced in the reaction. A scientist makes zinc iodide by reacting zinc with iodine. Dissolve the iodine in ethanol. Add an excess of. A Scientist Produces Zinc Iodide This Is The Method Used.
From www.youtube.com
Synthesis of Zinc Iodide Amazing Chemistry Experiment Partha Sir's A Scientist Produces Zinc Iodide This Is The Method Used 1 write the balanced chemical equation for the reaction between zinc and iodine: Weigh 0.500 g of iodine. Weigh 0.500 g of iodine. Add an excess of zinc. Dissolve the iodine in ethanol. Zn + i 2 zni 2 relative atomic masses (m r): This is the method used. A scientist makes zinc iodide by reacting zinc with iodine. Dissolve. A Scientist Produces Zinc Iodide This Is The Method Used.