Heat Capacity Of Water Lab . The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. In the first experiment we investigated the specific heat of water. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. permanent link for this species. there are different ways to determine the specific heat capacity of water. The water was heated by use of an electric. Use this link for bookmarking this species for future reference. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the heat capacity of ice is twice as high as that of most solids; the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. In this required practical activity it is important.
from www.chegg.com
In the first experiment we investigated the specific heat of water. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. the heat capacity of ice is twice as high as that of most solids; The water was heated by use of an electric. there are different ways to determine the specific heat capacity of water. In this required practical activity it is important. permanent link for this species. Use this link for bookmarking this species for future reference. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it.
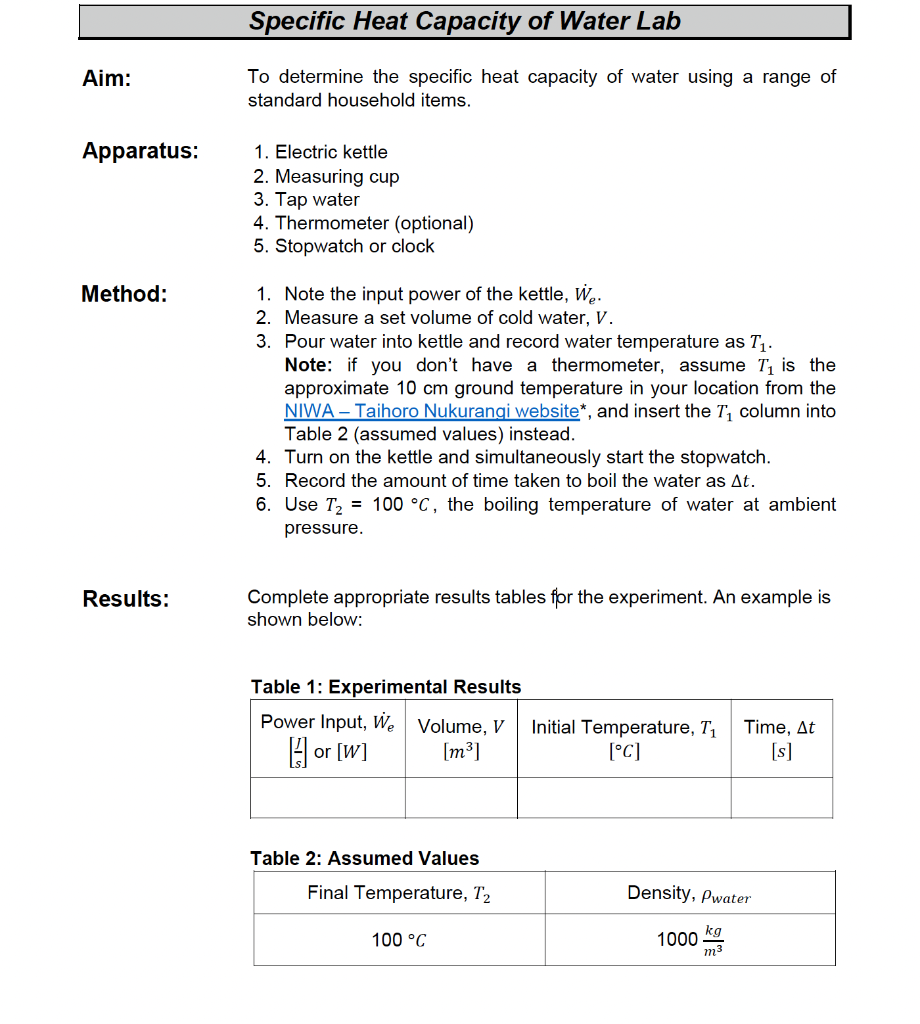
Solved Specific Heat Capacity of Water Lab Aim To determine
Heat Capacity Of Water Lab the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. In the first experiment we investigated the specific heat of water. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. The water was heated by use of an electric. Use this link for bookmarking this species for future reference. In this required practical activity it is important. permanent link for this species. the heat capacity of ice is twice as high as that of most solids; there are different ways to determine the specific heat capacity of water. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Capacity Of Water Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. In the first experiment we investigated the specific heat of water. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. The water was heated by use of. Heat Capacity Of Water Lab.
From www.scribd.com
A2 Physics Practicals 56873595 Practical 10 Specific Heat Capacity of a Liquid Heat Capacity Of Water Lab Use this link for bookmarking this species for future reference. there are different ways to determine the specific heat capacity of water. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. In this required practical activity it is important. In the first experiment we investigated. Heat Capacity Of Water Lab.
From mungfali.com
Specific Heat Capacity Of Water Experiment Heat Capacity Of Water Lab Use this link for bookmarking this species for future reference. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. permanent link for this species. In the first experiment we investigated the specific heat of water. the goal of this experiment is to experimentally determine the specific heat capacity. Heat Capacity Of Water Lab.
From mungfali.com
Specific Heat Capacity Of Water Experiment Heat Capacity Of Water Lab the heat capacity of ice is twice as high as that of most solids; The water was heated by use of an electric. permanent link for this species. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. In this required practical activity it is important. in this experiment you will heat a. Heat Capacity Of Water Lab.
From www.askwillonline.com
Specific Heat Capacity and Latent Heat Experiments In Physics Ask Will Online Heat Capacity Of Water Lab the heat capacity of ice is twice as high as that of most solids; In the first experiment we investigated the specific heat of water. In this required practical activity it is important. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. in this experiment you will heat. Heat Capacity Of Water Lab.
From www.markedbyteachers.com
Finding a material's specific heat capacity GCSE Science Marked by Heat Capacity Of Water Lab the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. The water was heated by use of an electric. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. Use this link for bookmarking this species for future. Heat Capacity Of Water Lab.
From www.youtube.com
Specific heat capacity YouTube Heat Capacity Of Water Lab The water was heated by use of an electric. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. permanent link for this species. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table. Heat Capacity Of Water Lab.
From keystagewiki.com
GCSE Physics Required Practical Determining Specific Heat Capacity Key Stage Wiki Heat Capacity Of Water Lab there are different ways to determine the specific heat capacity of water. permanent link for this species. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. In the first experiment we investigated the specific heat of water. the heat capacity of ice is twice as high as. Heat Capacity Of Water Lab.
From www.youtube.com
Heat capacity of soil vs. water LAB YouTube Heat Capacity Of Water Lab In this required practical activity it is important. The water was heated by use of an electric. there are different ways to determine the specific heat capacity of water. In the first experiment we investigated the specific heat of water. the heat capacity of ice is twice as high as that of most solids; the heat capacity. Heat Capacity Of Water Lab.
From studylib.net
1.3 Specific Heat Capacity Heat Capacity Of Water Lab In the first experiment we investigated the specific heat of water. the heat capacity of ice is twice as high as that of most solids; in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. The water was heated by use of an electric. permanent. Heat Capacity Of Water Lab.
From ch301.cm.utexas.edu
heating curve Heat Capacity Of Water Lab Use this link for bookmarking this species for future reference. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. there are different ways to determine the specific heat capacity of water. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. In the first experiment. Heat Capacity Of Water Lab.
From sractclx.blogspot.com
Specific Heat Capacity Water / schoolphysics The specific heat capacity (cp) of Heat Capacity Of Water Lab In this required practical activity it is important. Use this link for bookmarking this species for future reference. permanent link for this species. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. the heat capacity of a substance describes how its temperature changes as. Heat Capacity Of Water Lab.
From slidetodoc.com
MEASUREMENT OF THE SPECIFIC HEAT CAPACITY OF WATER Heat Capacity Of Water Lab The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. Use this link for bookmarking this species for future reference. the heat capacity of ice is twice as high as that of most solids; In the first experiment we investigated the specific heat of water. The water was heated by use of an electric. . Heat Capacity Of Water Lab.
From studylib.net
PHYSICS LAB Specific heat capacity of water Heat Capacity Of Water Lab The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. the heat capacity of ice is twice as high as that of most solids; there are different ways to determine the. Heat Capacity Of Water Lab.
From studylib.net
Specific heat capacity Heat Capacity Of Water Lab permanent link for this species. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. The water was heated by use of an electric. In this required practical activity it is important.. Heat Capacity Of Water Lab.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Heat Capacity Of Water Lab the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. Use this link for bookmarking this species for future reference. there are different ways to determine the specific heat capacity of water. In this required practical activity it is important. In the first experiment we investigated. Heat Capacity Of Water Lab.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Heat Capacity Of Water Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. permanent link for this species. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. there are different ways to determine the specific heat capacity of water. In this required practical. Heat Capacity Of Water Lab.
From ar.inspiredpencil.com
Heat Capacity Of Water Heat Capacity Of Water Lab In this required practical activity it is important. The water was heated by use of an electric. permanent link for this species. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. In the first experiment we investigated the specific heat of water. the goal of this experiment is. Heat Capacity Of Water Lab.
From ar.inspiredpencil.com
Heat Capacity Of Water Heat Capacity Of Water Lab In the first experiment we investigated the specific heat of water. permanent link for this species. Use this link for bookmarking this species for future reference. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. The water was heated by use of an electric. . Heat Capacity Of Water Lab.
From www.chegg.com
Solved Specific Heat Capacity of Water Lab Aim To determine Heat Capacity Of Water Lab the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. permanent link for this species. Use this link for bookmarking this species for future reference. In the. Heat Capacity Of Water Lab.
From sractclx.blogspot.com
Specific Heat Capacity Water / schoolphysics The specific heat capacity (cp) of Heat Capacity Of Water Lab there are different ways to determine the specific heat capacity of water. In this required practical activity it is important. The water was heated by use of an electric. the heat capacity of ice is twice as high as that of most solids; the goal of this experiment is to experimentally determine the specific heat capacity of. Heat Capacity Of Water Lab.
From www.youtube.com
Specific Heat Capacity of Water Physics Experiment YouTube Heat Capacity Of Water Lab The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. The water was heated by use of an electric. there are different ways to determine the specific heat capacity of water. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. in this experiment you. Heat Capacity Of Water Lab.
From studylib.net
Lab 11b Specific Heat & Heat Capacity Heat Capacity Of Water Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. In the first experiment we investigated the specific heat of water. the heat capacity of ice is twice as high as that of most solids; the goal of this experiment is to experimentally determine the. Heat Capacity Of Water Lab.
From www.slideserve.com
PPT Measurement of the specific heat capacity of a liquid by an electrical method PowerPoint Heat Capacity Of Water Lab the heat capacity of ice is twice as high as that of most solids; The water was heated by use of an electric. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. there are different ways to determine the specific heat capacity of water. in this experiment. Heat Capacity Of Water Lab.
From cuagodep.net
What Is The Molar Heat Capacity Of Water Unraveling Its Thermal Mysteries Heat Capacity Of Water Lab the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. In this required practical activity it is important. there are different ways to determine the specific heat capacity of water. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. permanent link for this species.. Heat Capacity Of Water Lab.
From www.youtube.com
Specific Heat Capacity of Water Experiment RESULTS GCSE Physics Required Practical YouTube Heat Capacity Of Water Lab the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. there are different ways to determine the specific heat capacity of water. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. The heat capacity of liquid. Heat Capacity Of Water Lab.
From studylib.net
LAB 3 SPECIFIC HEAT CAPACITY (method of Heat Capacity Of Water Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the heat capacity of ice is twice as high as that of most solids; The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. In the first experiment we investigated the specific. Heat Capacity Of Water Lab.
From physicsexperiments.eu
Experimental determination of Specific Heat of Water — Collection of Experiments Heat Capacity Of Water Lab the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. The water was heated by use of an electric. In this required practical activity it is important. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. . Heat Capacity Of Water Lab.
From studymind.co.uk
Heat Capacity Study Mind Heat Capacity Of Water Lab In the first experiment we investigated the specific heat of water. permanent link for this species. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. In this required practical activity it is important. The water was heated by use of an electric. there are. Heat Capacity Of Water Lab.
From gcsephysicsninja.com
38. Experiment to measure specific heat capacity Heat Capacity Of Water Lab there are different ways to determine the specific heat capacity of water. the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. . Heat Capacity Of Water Lab.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download ID3439007 Heat Capacity Of Water Lab the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. permanent link for this species. The heat capacity of liquid water, 4.184 j/(g•°c),. Heat Capacity Of Water Lab.
From www.tec-science.com
Specific heat capacity of selected substances tecscience Heat Capacity Of Water Lab permanent link for this species. there are different ways to determine the specific heat capacity of water. the heat capacity of ice is twice as high as that of most solids; Use this link for bookmarking this species for future reference. the heat capacity of a substance describes how its temperature changes as it absorbs or. Heat Capacity Of Water Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity Heat Capacity Of Water Lab the goal of this experiment is to experimentally determine the specific heat capacity of water and compare it with the table value. Use this link for bookmarking this species for future reference. there are different ways to determine the specific heat capacity of water. in this experiment you will heat a known mass of a metal to. Heat Capacity Of Water Lab.
From mungfali.com
Specific Heat Capacity Of Water Experiment Heat Capacity Of Water Lab the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. In this required practical activity it is important. The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. The water was heated by use of an electric. permanent link for this species. in this experiment. Heat Capacity Of Water Lab.
From alevelphysicsnotes.com
Mr Toogood Physics Specific heat capacity Heat Capacity Of Water Lab The heat capacity of liquid water, 4.184 j/(g•°c), is one of the highest known. Use this link for bookmarking this species for future reference. the heat capacity of a substance describes how its temperature changes as it absorbs or releases heat, it. In the first experiment we investigated the specific heat of water. there are different ways to. Heat Capacity Of Water Lab.