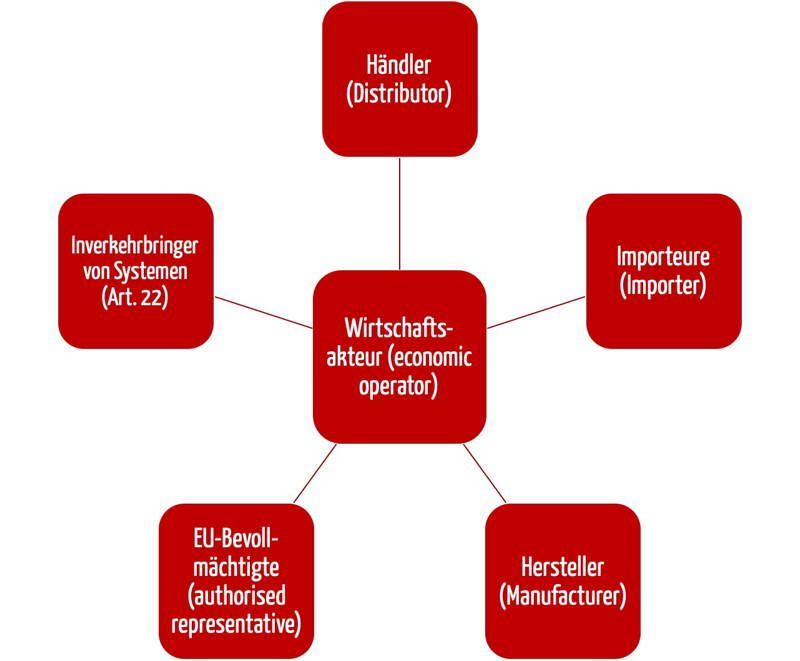
Distributor Requirements Under Mdr . Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! When making a device available on the market, distributors shall, in the context of their. These entities must comply with article 14 of the regulations and any applicable national registration What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while
from www.johner-institute.com
What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on the market, distributors shall, in the context of their. Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. These entities must comply with article 14 of the regulations and any applicable national registration
MDR Distributor Requirements (that also affect the manufacturers)
Distributor Requirements Under Mdr In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! When making a device available on the market, distributors shall, in the context of their. These entities must comply with article 14 of the regulations and any applicable national registration What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor.
From mdlaw.eu
MDR Distributor/Importer/Manufacturer Quality Agreement · MDlaw Distributor Requirements Under Mdr In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. These entities must comply with article 14 of the regulations and any applicable national registration Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels.. Distributor Requirements Under Mdr.
From www.switchcomp.com
/ Find our Distributor Switch Components Inc Distributor Requirements Under Mdr These entities must comply with article 14 of the regulations and any applicable national registration Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Distributors should verify that the devices have been ce marked, that an eu declaration. Distributor Requirements Under Mdr.
From www.greenlight.guru
What are the Distributor Requirements under EU MDR and IVDR? Distributor Requirements Under Mdr For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. These entities must comply with article 14 of the. Distributor Requirements Under Mdr.
From mdlaw.eu
MDR Distributor/Importer/Manufacturer Quality Agreement · MDlaw Distributor Requirements Under Mdr The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on the market, distributors shall, in. Distributor Requirements Under Mdr.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Distributor Requirements Under Mdr When making a device available on the market, distributors shall, in the context of their. These entities must comply with article 14 of the regulations and any applicable national registration In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. Your analysis of the mdr’s impact on distributor. Distributor Requirements Under Mdr.
From medicaldevicehq.com
Education and training requirements for the PRRC under the MDR Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. In general, the mdr and the. Distributor Requirements Under Mdr.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. These entities must comply with article 14 of the regulations and any applicable national registration Your analysis of the mdr’s. Distributor Requirements Under Mdr.
From www.avanti-europe.ch
What you need to know about the MDR classification rules Distributor Requirements Under Mdr These entities must comply with article 14 of the regulations and any applicable national registration In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on. Distributor Requirements Under Mdr.
From decomplix.com
Which MDR requirements apply to distributors of medical devices? Distributor Requirements Under Mdr When making a device available on the market, distributors shall, in the context of their. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. For instance, a community pharmacy. Distributor Requirements Under Mdr.
From intellisoft.io
EU Regulation Transitioning from the MDD to MDR Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. These entities must comply with article 14 of the regulations and any applicable national registration When making a device available on the market, distributors. Distributor Requirements Under Mdr.
From flamlabelthema.netlify.app
Eu Mdr Declaration Of Conformity Template Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. These entities must. Distributor Requirements Under Mdr.
From supplychain.enchange.com
15 Essentials to Drive FMCG Distribution & Execution Distributor Requirements Under Mdr When making a device available on the market, distributors shall, in the context of their. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. In general, the mdr and the ivdr retain. Distributor Requirements Under Mdr.
From www.rqmplus.com
Economic Operators EU MDR and IVDR Requirements Distributor Requirements Under Mdr For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Your analysis of the mdr’s impact. Distributor Requirements Under Mdr.
From acf.com.tr
OEM PLM under MDR. Which model you will choose? Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. In general, the mdr and the. Distributor Requirements Under Mdr.
From www.vrogue.co
Canadian Device Labeling Requirements Ce Mark Package vrogue.co Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while These entities must comply with article 14 of the regulations and any applicable national registration Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. When making a device available. Distributor Requirements Under Mdr.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Distributor Requirements Under Mdr These entities must comply with article 14 of the regulations and any applicable national registration When making a device available on the market, distributors shall, in the context of their. In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. The mdr and ivdr regulations present challenges for. Distributor Requirements Under Mdr.
From growthimports.eu
Distributor and Importer MDR/IVDR Regulations Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. In general, the mdr and the ivdr retain all the requirements. Distributor Requirements Under Mdr.
From www.vrogue.co
Eu Mdr Medical Device Labeling Requirements A Complet vrogue.co Distributor Requirements Under Mdr In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. For instance, a community pharmacy that buys and sells medical face masks falls within the. Distributor Requirements Under Mdr.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Distributor Requirements Under Mdr In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while The mdr. Distributor Requirements Under Mdr.
From www.greenlight.guru
Ultimate Guide to Device Class Requirements under EU MDR Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. When making a device available on the market, distributors shall, in the context of their. Your analysis of the mdr’s impact. Distributor Requirements Under Mdr.
From www.slideteam.net
Criteria For Selecting Distribution Channel For Effective Sales Distributor Requirements Under Mdr These entities must comply with article 14 of the regulations and any applicable national registration When making a device available on the market, distributors shall, in the context of their. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system. Distributor Requirements Under Mdr.
From www.nasiol.com
How to A Nasiol Distributor Nasiol Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while These entities must comply with article 14 of the regulations and any applicable national registration Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! Distributors should verify that the devices have been ce marked, that an eu declaration. Distributor Requirements Under Mdr.
From mdlaw.eu
MDR Checklist General Safety and Performance Requirements (Annex I Distributor Requirements Under Mdr Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of. Distributor Requirements Under Mdr.
From www.johner-institute.com
MDR Distributor Requirements (that also affect the manufacturers) Distributor Requirements Under Mdr The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. These entities must comply with article 14 of the regulations and any applicable national registration What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on the market, distributors shall,. Distributor Requirements Under Mdr.
From www.avanti-europe.ch
QMS requirements for distributors under MDR and IVDR Distributor Requirements Under Mdr The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. When making a device available on the market, distributors shall, in the context of their. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. In general, the mdr and the ivdr retain. Distributor Requirements Under Mdr.
From qbdgroup.com
CE Approval for Medical Devices under MDR key requirements per device Distributor Requirements Under Mdr In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on the market, distributors shall, in the context of their. Distributors should verify that the devices. Distributor Requirements Under Mdr.
From www.gbu-presnenskij.ru
MDR Checklist General Safety And Performance Requirements, 47 OFF Distributor Requirements Under Mdr These entities must comply with article 14 of the regulations and any applicable national registration In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. Your analysis of the mdr’s. Distributor Requirements Under Mdr.
From medenvoyglobal.com
eIFU Requirements Under the MDR Distributor Requirements Under Mdr The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! These entities must comply with article 14 of the. Distributor Requirements Under Mdr.
From www.1med.ch
Clinical data and clinical evidence requirements under MDR free Expert Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on the market, distributors shall, in the context of their. These entities must comply with article 14 of the regulations and any applicable national registration The mdr and ivdr regulations present challenges for all economic operators and it. Distributor Requirements Under Mdr.
From www.celegence.com
Insights into PMCF Requirements under EU MDR Celegence Distributor Requirements Under Mdr Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while When making a device available on the market, distributors shall, in the context of their. These entities must comply with. Distributor Requirements Under Mdr.
From www.rqmplus.com
Mastering the Transition 5 Essential Tips for EU MDR Compliance Distributor Requirements Under Mdr In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! Distributors should verify that the devices have been ce marked, that an eu declaration of conformity has been drawn up, and that labels. What. Distributor Requirements Under Mdr.
From www.greenlight.guru
EU MDR and IVDR Distributor Requirements [Overview] Distributor Requirements Under Mdr For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. In general, the mdr and the ivdr retain all the requirements of the directives, while adding some new requirements of their own. These entities must comply with article 14 of the regulations and any applicable national registration Distributors should verify that. Distributor Requirements Under Mdr.
From www.scribd.com
Technical Documentation Requirements Under MDR (Including Requirements Distributor Requirements Under Mdr What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. These entities must comply with article 14 of the regulations and. Distributor Requirements Under Mdr.
From www.argosmultilingual.com
New Requirements for Software Under MDR and IVDR Argos Multilingual Distributor Requirements Under Mdr For instance, a community pharmacy that buys and sells medical face masks falls within the definition of a distributor. What key strategies do you recommend for distributors to effectively implement a comprehensive quality management system while Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! When making a device available on the market, distributors shall,. Distributor Requirements Under Mdr.
From easymedicaldevice.com
We help you under EU Technical Documentation Distributor Requirements Under Mdr When making a device available on the market, distributors shall, in the context of their. The mdr and ivdr regulations present challenges for all economic operators and it is important to act early. Your analysis of the mdr’s impact on distributor requirements is both clear and insightful! For instance, a community pharmacy that buys and sells medical face masks falls. Distributor Requirements Under Mdr.