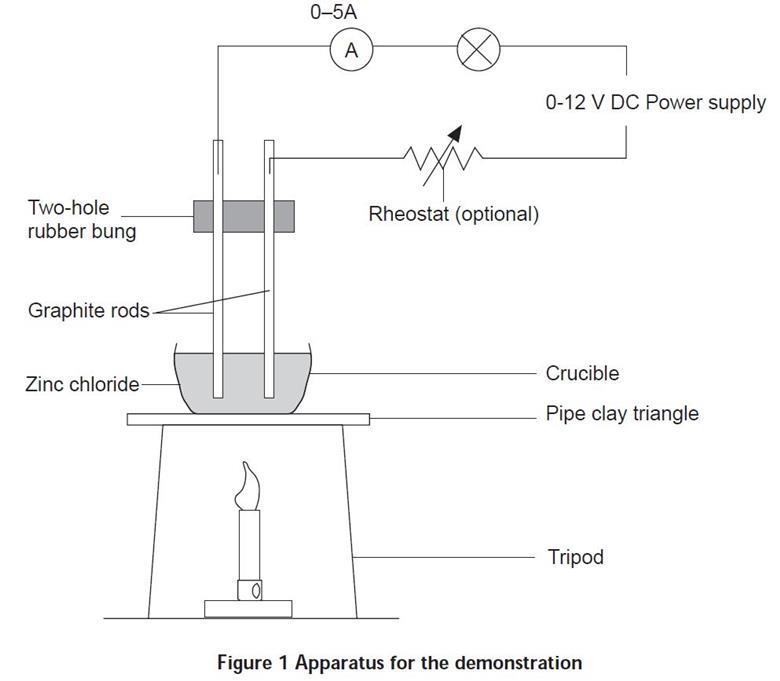
Zinc Chloride Formula Electrolysis . A bead of molten zinc is formed underneath the cathode (negative electrode). The chemical equation for this reaction is given by: The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. This demonstration shows that an ionic salt conducts electricity. Equations the zinc ions are reduced to zinc atoms by gaining. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Zinc chloride must be heated until it is molten before it will conduct. Zn + 2hcl → zncl2 + h2. This @theelkchemist gcse short takes your through tips and hints relating to the. Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. Zinc chloride, anhydrous and its hydrates, are. The chemical equation for this reaction is given by: Electrolysis of molten zinc chloride. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride.
from edu.rsc.org
Zinc chloride, anhydrous and its hydrates, are. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. Equations the zinc ions are reduced to zinc atoms by gaining. The chemical equation for this reaction is given by: This @theelkchemist gcse short takes your through tips and hints relating to the. Zn + 2hcl → zncl2 + h2. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride.
Electrolysis of molten zinc chloride Resource RSC Education
Zinc Chloride Formula Electrolysis The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. The chemical equation for this reaction is given by: The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. This demonstration shows that an ionic salt conducts electricity. Electrolysis of molten zinc chloride. The chemical equation for this reaction is given by: Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. This @theelkchemist gcse short takes your through tips and hints relating to the. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Zinc chloride must be heated until it is molten before it will conduct. Zn + 2hcl → zncl2 + h2.
From www.911metallurgist.com
Zinc Electrolysis Zinc Chloride Formula Electrolysis The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. Equations the zinc ions are reduced to zinc atoms by gaining. Electrolysis of molten zinc chloride. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Zinc can be extracted from zinc oxide by heating with carbon or. Zinc Chloride Formula Electrolysis.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Zinc Chloride Formula Electrolysis A bead of molten zinc is formed underneath the cathode (negative electrode). The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Zinc chloride, anhydrous and its hydrates, are. Zn + 2hcl → zncl2 + h2. Electrolysis of molten zinc chloride. Zinc chloride also offers a safer alternative to lead bromide. Zinc Chloride Formula Electrolysis.
From www.shutterstock.com
122 Zinc Chloride Images, Stock Photos & Vectors Shutterstock Zinc Chloride Formula Electrolysis Equations the zinc ions are reduced to zinc atoms by gaining. The chemical equation for this reaction is given by: The chemical equation for this reaction is given by: Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. Electrolysis of molten zinc chloride.. Zinc Chloride Formula Electrolysis.
From www.youtube.com
How to Write the Formula for Zinc chloride (ZnCl2) YouTube Zinc Chloride Formula Electrolysis Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Electrolysis of molten zinc chloride. The chemical equation for this reaction is given by: Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. Zinc chloride, anhydrous. Zinc Chloride Formula Electrolysis.
From www.tessshebaylo.com
Electrolysis Of Salt Water Half Equations Tessshebaylo Zinc Chloride Formula Electrolysis Equations the zinc ions are reduced to zinc atoms by gaining. This @theelkchemist gcse short takes your through tips and hints relating to the. Zn + 2hcl → zncl2 + h2. The chemical equation for this reaction is given by: The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Understand. Zinc Chloride Formula Electrolysis.
From www.numerade.com
SOLVED Molten zinc chloride can be electrolysed. Describe how this Zinc Chloride Formula Electrolysis The chemical equation for this reaction is given by: Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in. Zinc Chloride Formula Electrolysis.
From www.911metallurgist.com
Zinc Electrolysis Zinc Chloride Formula Electrolysis Electrolysis of molten zinc chloride. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. This demonstration shows that an ionic salt conducts electricity. Understand how molten zinc chloride conducts electricity. Zinc Chloride Formula Electrolysis.
From www.pakistanchemical.com
Zinc Chloride PAKISTAN CHEMICAL Zinc Chloride Formula Electrolysis A bead of molten zinc is formed underneath the cathode (negative electrode). The chemical equation for this reaction is given by: Equations the zinc ions are reduced to zinc atoms by gaining. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. This @theelkchemist gcse short takes your through tips and hints relating to the.. Zinc Chloride Formula Electrolysis.
From www.shutterstock.com
Zinc Chloride Formula Handwritten Chemical Formula Stock Illustration Zinc Chloride Formula Electrolysis Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Zinc chloride. Zinc Chloride Formula Electrolysis.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Zinc Chloride Formula Electrolysis Zinc chloride, anhydrous and its hydrates, are. This demonstration shows that an ionic salt conducts electricity. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. The chemical equation for this reaction is given by: Zinc chloride must be heated until it is molten before it will conduct. Zinc can be extracted from. Zinc Chloride Formula Electrolysis.
From www.researchgate.net
Schematic illustration of a) the slow electrolysis of the zinc powder Zinc Chloride Formula Electrolysis A bead of molten zinc is formed underneath the cathode (negative electrode). This @theelkchemist gcse short takes your through tips and hints relating to the. Electrolysis of molten zinc chloride. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. Equations the zinc ions. Zinc Chloride Formula Electrolysis.
From www.elevise.co.uk
C4 K) Electrolysis Part 2 AQA Combined Science Trilogy Elevise Zinc Chloride Formula Electrolysis This demonstration shows that an ionic salt conducts electricity. This @theelkchemist gcse short takes your through tips and hints relating to the. The chemical equation for this reaction is given by: Equations the zinc ions are reduced to zinc atoms by gaining. Electrolysis of molten zinc chloride. Zinc can be extracted from zinc oxide by heating with carbon or from. Zinc Chloride Formula Electrolysis.
From www.youtube.com
IC15 Electrolysis of sodium chloride solution YouTube Zinc Chloride Formula Electrolysis The chemical equation for this reaction is given by: In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when. Zinc Chloride Formula Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Zinc Chloride Formula Electrolysis Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. The chemical equation for this reaction is given by:. Zinc Chloride Formula Electrolysis.
From www.youtube.com
How to Balance NaCl = Na + Cl2 (Electrolysis of Sodium chloride ) YouTube Zinc Chloride Formula Electrolysis Zinc chloride, anhydrous and its hydrates, are. Zn + 2hcl → zncl2 + h2. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. The chemical equation for this reaction is given by: This @theelkchemist gcse short takes your through. Zinc Chloride Formula Electrolysis.
From www.youtube.com
Electrolysis of Molten Sodium Chloride YouTube Zinc Chloride Formula Electrolysis Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. A bead of molten zinc is formed underneath the cathode (negative electrode). Equations the zinc ions are reduced to zinc atoms by gaining. Zinc chloride is an inorganic chemical compound. Zinc Chloride Formula Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk Zinc Chloride Formula Electrolysis Electrolysis of molten zinc chloride. The chemical equation for this reaction is given by: In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. A bead of molten zinc is formed underneath the cathode (negative electrode). Zinc can be extracted from zinc oxide by heating with carbon or. Zinc Chloride Formula Electrolysis.
From edu.svet.gob.gt
Molecular Formula Zinc Chloride Chemical Structure Stock Zinc Chloride Formula Electrolysis The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. The chemical equation for this reaction is given by: A bead of molten zinc is formed underneath the cathode (negative electrode). The chemical equation for this reaction is given by: Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. Zinc chloride. Zinc Chloride Formula Electrolysis.
From www.shutterstock.com
Molten Mass Electrolysis Of Nacl Sodium Chloride Stock Vector Zinc Chloride Formula Electrolysis Zinc chloride must be heated until it is molten before it will conduct. The chemical equation for this reaction is given by: Zn + 2hcl → zncl2 + h2. Zinc chloride, anhydrous and its hydrates, are. This demonstration shows that an ionic salt conducts electricity. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of. Zinc Chloride Formula Electrolysis.
From issuu.com
6. Zinc Chloride Electrolysis by Aniqa Masroor Issuu Zinc Chloride Formula Electrolysis Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. Zinc chloride, anhydrous and its hydrates, are. Zinc chloride must be heated until it is molten before it will conduct. The chemical equation for this reaction is given by: This demonstration shows that an ionic salt conducts electricity. Zinc can be extracted from zinc oxide. Zinc Chloride Formula Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps Zinc Chloride Formula Electrolysis Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. The chemical equation for this reaction is given by: Electrolysis of molten zinc chloride. The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. Equations the zinc ions are reduced to zinc atoms by gaining. Zinc chloride also offers a safer alternative to lead. Zinc Chloride Formula Electrolysis.
From www.youtube.com
Equation for ZnCl2 + H2O (Zinc chloride + Water) YouTube Zinc Chloride Formula Electrolysis The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. Equations the zinc ions are reduced to zinc atoms by gaining. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n. Zinc Chloride Formula Electrolysis.
From studyrocket.co.uk
Electrolysis GCSE Chemistry Science) OCR Revision Study Zinc Chloride Formula Electrolysis Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. Equations the zinc ions are reduced to zinc atoms by gaining. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. This @theelkchemist gcse short takes your through tips and hints. Zinc Chloride Formula Electrolysis.
From askfilo.com
zinc chloride(IV). 30. Correct formula of tetraamminechloridonitroplatinu.. Zinc Chloride Formula Electrolysis Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. Zinc chloride must be heated until it is molten before it will conduct. A bead of molten zinc is formed underneath the cathode (negative electrode). The chemical equation for this reaction is given by: Zinc chloride also offers a safer alternative to lead bromide for. Zinc Chloride Formula Electrolysis.
From www.youtube.com
Electrolysis of Molten Sodium Chloride (Beta Version) YouTube Zinc Chloride Formula Electrolysis Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. The chemical equation for this reaction is given by: This demonstration shows that an ionic salt conducts. Zinc Chloride Formula Electrolysis.
From www.tessshebaylo.com
Salt Water Electrolysis Equation Tessshebaylo Zinc Chloride Formula Electrolysis Zinc chloride must be heated until it is molten before it will conduct. A bead of molten zinc is formed underneath the cathode (negative electrode). Electrolysis of molten zinc chloride. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Zinc chloride also offers a safer alternative to lead bromide for. Zinc Chloride Formula Electrolysis.
From www.vrogue.co
Sodium Chloride Electrolysis Equation vrogue.co Zinc Chloride Formula Electrolysis Equations the zinc ions are reduced to zinc atoms by gaining. Electrolysis of molten zinc chloride. The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. The chemical equation for this reaction is given by: Zinc chloride also offers a safer alternative. Zinc Chloride Formula Electrolysis.
From www.dreamstime.com
Molecular Formula of Zinc Chloride Stock Illustration Illustration of Zinc Chloride Formula Electrolysis Zinc chloride, anhydrous and its hydrates, are. Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. The chemical equation for this reaction is given by: Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Electrolysis. Zinc Chloride Formula Electrolysis.
From camachem.com
What is Zinc Chloride and How to Buy Zinc Chloride? Zinc Chloride Formula Electrolysis Zinc chloride must be heated until it is molten before it will conduct. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. Electrolysis of molten zinc chloride. The chemical equation for this reaction is given. Zinc Chloride Formula Electrolysis.
From edu.rsc.org
Electrolysis of molten zinc chloride Resource RSC Education Zinc Chloride Formula Electrolysis Electrolysis of molten zinc chloride. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. The chemical equation for this reaction is given by: Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. Zinc chloride must be heated until it is molten before it will conduct. Zinc. Zinc Chloride Formula Electrolysis.
From www.nagwa.com
Question Video Determining the Equation That Shows the Reaction at the Zinc Chloride Formula Electrolysis Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. The chemical equation for this reaction is given by: Zinc chloride is an inorganic chemical compound with the formula zncl 2 · n h 2 o, with n ranging from 0 to 4.5, forming hydrates. Electrolysis of molten zinc chloride. This demonstration shows. Zinc Chloride Formula Electrolysis.
From sierrauru.blogspot.com
Electrolysis Of Sodium Chloride Zinc Chloride Formula Electrolysis Electrolysis of molten zinc chloride. In order to obtain a hydrated form of the compound, hydrochloric acid can be used to treat zinc instead of hydrogen chloride. The chemical equation for this reaction is given by: Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. Zinc chloride, anhydrous and its hydrates, are.. Zinc Chloride Formula Electrolysis.
From coitivisorp.weebly.com
Electrolysisofcopperchlorideobservations Zinc Chloride Formula Electrolysis Equations the zinc ions are reduced to zinc atoms by gaining. The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. Zinc chloride, anhydrous and its hydrates, are. Zinc chloride also offers a safer alternative to lead bromide for demonstrating the electrolysis of molten salts. The chemical equation for this reaction is given by: The electrolysis of. Zinc Chloride Formula Electrolysis.
From www.pw.live
Zinc Chloride Formula Zinc Chloride Formula Electrolysis Zinc can be extracted from zinc oxide by heating with carbon or from zinc chloride by electrolysis. Zinc chloride must be heated until it is molten before it will conduct. The electrolysis of zinc chloride shows how an ionic salt will conduct electricity when molten, but not when solid. Equations the zinc ions are reduced to zinc atoms by gaining.. Zinc Chloride Formula Electrolysis.
From www.slideserve.com
PPT Do now! PowerPoint Presentation, free download ID3943397 Zinc Chloride Formula Electrolysis Zinc chloride must be heated until it is molten before it will conduct. Hydrochloric acid reacts with zinc sulphide to form zinc chloride and hydrogen sulphide. Understand how molten zinc chloride conducts electricity over solid zinc chloride, in this practical experiment. The demonstration uses zinc chloride, as this will melt at bunsen burner temperatures. This demonstration shows that an ionic. Zinc Chloride Formula Electrolysis.