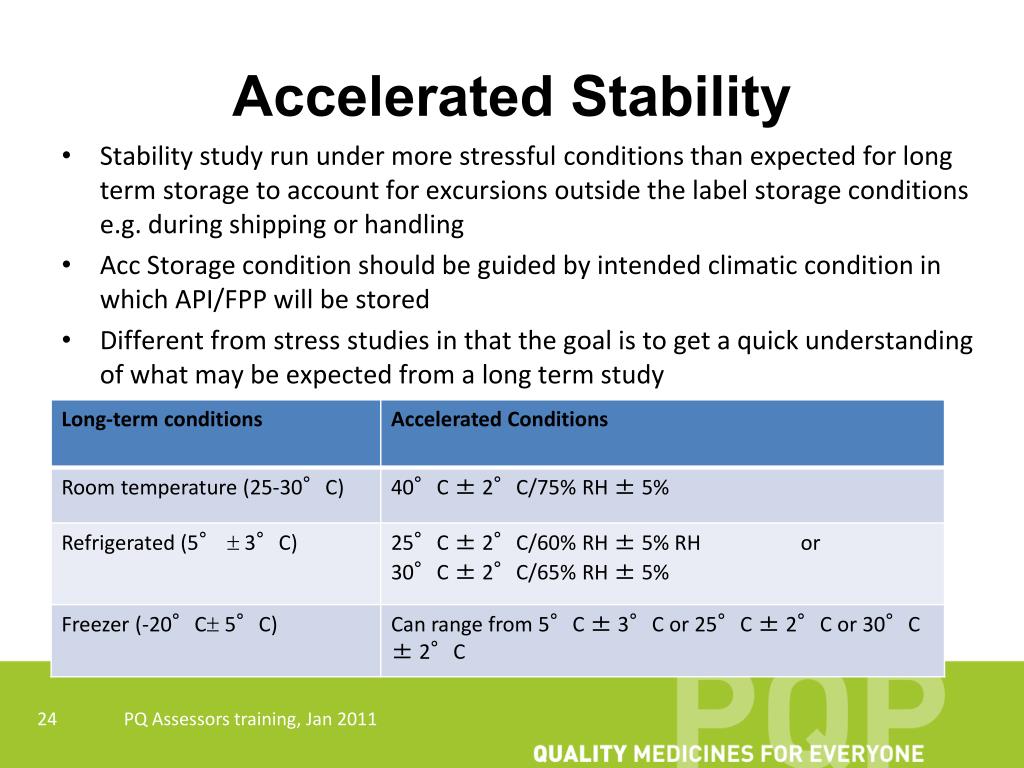
Stability Testing Zones . The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid.
from www.slideserve.com
This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid.
PPT Stability Testing PowerPoint Presentation, free download ID481423
Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are.
From www.researchgate.net
Comparison of the Global Stability Zones of the CCISMload system Stability Testing Zones The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The international council for harmonisation (ich) has established four distinct stability zones to guide the. Stability Testing Zones.
From www.slideshare.net
Ich guideline for stability testing Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. This document defines the stability data package for a new drug substance or drug product that is sufficient. Stability Testing Zones.
From www.pharmainform.com
Climatic Zones For Stability Studies Pharmainform Stability Testing Zones This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. This document defines the stability data package for a new drug substance or drug product that is sufficient. Stability Testing Zones.
From www.slideserve.com
PPT Drug Stability and Stabilization Techniques PowerPoint Stability Testing Zones The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence of how the quality of. Stability Testing Zones.
From www.pharmasopworld.com
Climatic Zones Stability testing of active pharmaceutical ingredients Stability Testing Zones The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. This document defines the stability data package for a new drug substance or drug product. Stability Testing Zones.
From www.slideshare.net
Ich guidelines for stability studies 1 Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing. Stability Testing Zones.
From insideofpharma.blogspot.com
Pharma Knowledge Stability and Stability Zone Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. This document defines the stability data package for a new drug substance or drug product. Stability Testing Zones.
From pdfslide.net
(PDF) Climate Zones Classification · Climate Zones Classification DRUG Stability Testing Zones This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. The international council for harmonisation (ich) has established four distinct stability zones to. Stability Testing Zones.
From www.slideserve.com
PPT STABILITY STUDIES PowerPoint Presentation ID217866 Stability Testing Zones The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. The design of the stability testing programme should take into. Stability Testing Zones.
From latampharmara.com
Stability Studies Regulatory Affairs in Latin America Stability Testing Zones The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Know different climatic zones by ich in the world for. Stability Testing Zones.
From www.slideshare.net
Stability zones and ich guideline q5c Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. The international council for harmonisation (ich) has established four distinct stability zones. Stability Testing Zones.
From www.slideserve.com
PPT Regulatory requirements on Medicine Stability Guidelines relevant Stability Testing Zones This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical. Stability Testing Zones.
From www.semanticscholar.org
[PDF] ON STABILITY STUDIES OF PHARMACEUTICAL PRODUCTS Semantic Scholar Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic. Stability Testing Zones.
From www.slideserve.com
PPT Stability Testing PowerPoint Presentation, free download ID481423 Stability Testing Zones This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean. Stability Testing Zones.
From q1scientific.com
A Q&A guide to stability storage Q1 Scientific Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. This document defines the stability data package for a new drug substance or drug product that is sufficient. Stability Testing Zones.
From www.slideshare.net
ICH Guidelines for stability testing Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i.. Stability Testing Zones.
From www.scribd.com
ICH Stability Zones and Testing Conditions PDF Stability Testing Zones The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability studies should include testing of those attributes of the drug substance that are. Stability Testing Zones.
From www.slideserve.com
PPT WHO Prequalification and API Requirements PowerPoint Presentation Stability Testing Zones The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The purpose of stability testing is to provide evidence of how the quality of an api. Stability Testing Zones.
From ccg-group.eu
Creating Zones of Stability Conscious Consulting Group Stability Testing Zones The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The design of the stability testing programme should take into account the intended market and the. Stability Testing Zones.
From www.az-biopharm.de
Stability testing and storage AZ Biopharm Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline provides recommendations on stability testing protocols including temperature, humidity and. Stability Testing Zones.
From www.slideshare.net
Ich guidelines for stability studies 1 Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. This document defines the stability data package for a new drug substance or drug product that. Stability Testing Zones.
From www.slideshare.net
ICH Guidelines for stability testing Stability Testing Zones This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the. Stability Testing Zones.
From www.slideserve.com
PPT DRUG STABILITY PowerPoint Presentation, free download ID12200727 Stability Testing Zones The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. This document defines the stability data package for a. Stability Testing Zones.
From insideofpharma.blogspot.com
Pharma Knowledge Stability and Stability Zone Stability Testing Zones This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This guideline provides recommendations on stability testing protocols including temperature,. Stability Testing Zones.
From www.slideshare.net
Ich guidelines for stability studies 2 Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. This document defines the stability data package for a new drug substance or drug product that. Stability Testing Zones.
From www.slideshare.net
Stability studies ICH Q1AQ1E Guidelines ppt Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic. Stability Testing Zones.
From seeko-sri.blogspot.com
SEEKO Stability Zones Stability Testing Zones The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing of drugs. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial. Stability Testing Zones.
From www.slideshare.net
ICH Guidelines for stability testing Stability Testing Zones This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage. Stability Testing Zones.
From www.researchgate.net
Mathieu stability diagram of the first stability zone near the working Stability Testing Zones This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. Know different climatic zones by ich in the world for. Stability Testing Zones.
From www.slideshare.net
Ich guidelines for stability studies 1 Stability Testing Zones This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product. Stability Testing Zones.
From www.researchgate.net
The stability zones for the agents from Fig. 8. For the training, the Stability Testing Zones The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. This document defines the stability data package for a new drug substance or drug product. Stability Testing Zones.
From www.slideshare.net
Stability zones and ich guideline q5c Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i. The international council for harmonisation (ich) has established four distinct stability zones to guide the pharmaceutical industry in the stability testing. Stability Testing Zones.
From www.scribd.com
Stability Zones of World PDF Temperate Climate Climate Stability Testing Zones Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. The design of the stability testing programme should take into account the intended market and the climatic conditions in the area in which the. The purpose of stability testing is to provide evidence of how the quality of an. Stability Testing Zones.
From q1scientific.com
Q1 Scientific Pharmaceutical Blog Series ICH Conditions Stability Testing Zones Know different climatic zones by ich in the world for stability conditions including temperate, mediterranean / subtropical, hot dry, hot humid. Stability studies should include testing of those attributes of the drug substance that are susceptible to change during storage and are. This guideline provides recommendations on stability testing protocols including temperature, humidity and trial duration for climatic zone i.. Stability Testing Zones.
From www.slideshare.net
Ich guidelines for stability studies 1 Stability Testing Zones The purpose of stability testing is to provide evidence of how the quality of an api or fpp varies with time under the influence of a variety. This document defines the stability data package for a new drug substance or drug product that is sufficient for a registration application within. The international council for harmonisation (ich) has established four distinct. Stability Testing Zones.