Magnesium Valence Electrons Atom . 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. 119 rows valence electrons: In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. An atom in group 1 has only a single valence electron. Elements from groups have preferred numbers of valence electrons. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds.
from www.alamy.com
Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Elements from groups have preferred numbers of valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. 119 rows valence electrons: This one valence electron is easily lost to form a positive ion with an #s^2p^6#. An atom in group 1 has only a single valence electron.
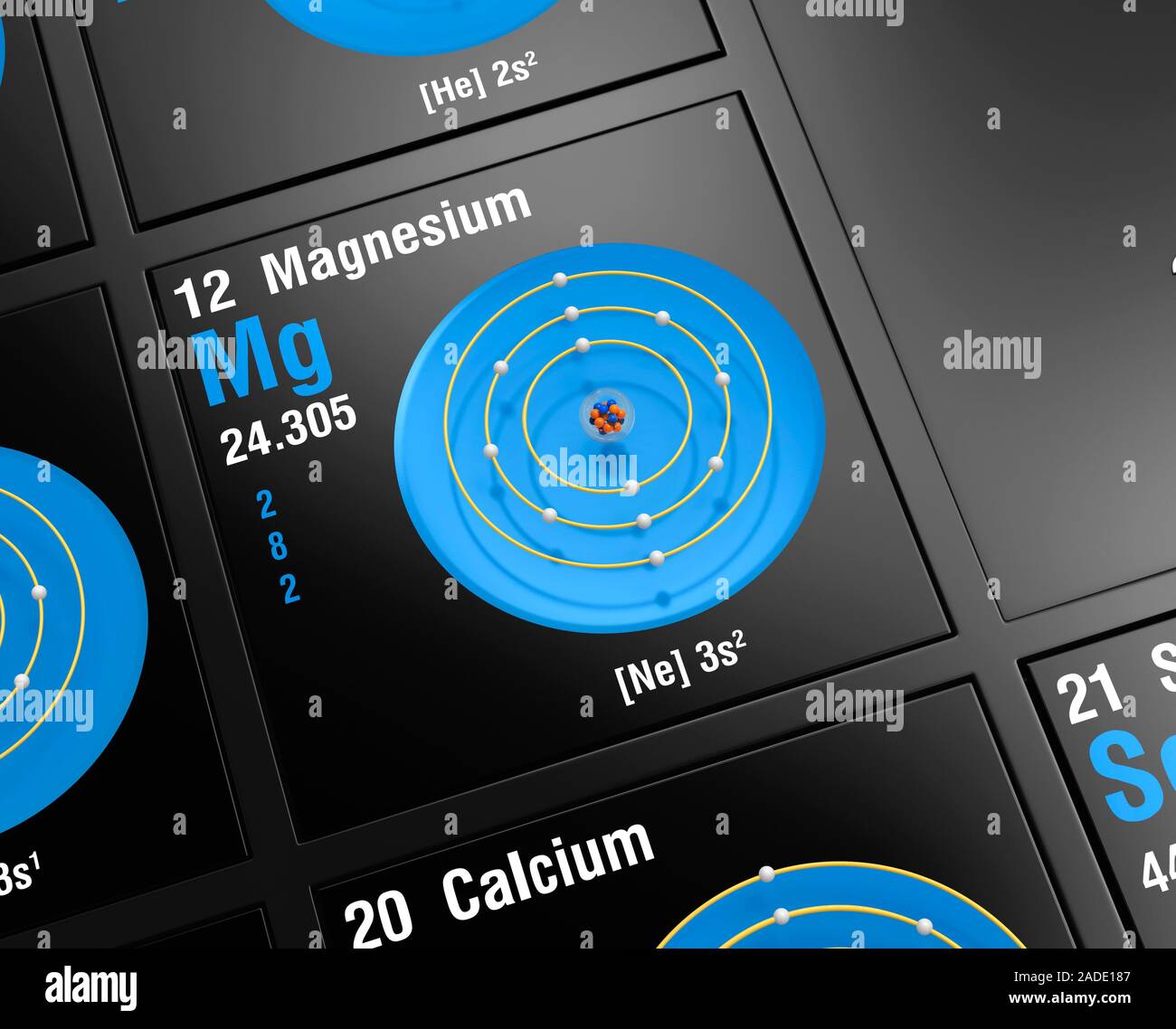
Diagram of the nuclear composition, electron configuration, and valence
Magnesium Valence Electrons Atom Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. An atom in group 1 has only a single valence electron. 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. This one valence electron is easily lost to form a positive ion with an #s^2p^6#.
From ar.inspiredpencil.com
Magnesium Electron Configuration Magnesium Valence Electrons Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. An atom in group 1 has only a single. Magnesium Valence Electrons Atom.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. 119 rows valence electrons: An atom in group 1 has only a single valence electron. Elements from groups have preferred numbers. Magnesium Valence Electrons Atom.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. In chemistry and physics, valence electrons are electrons in the outermost shell of. Magnesium Valence Electrons Atom.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Valence Electrons Atom Elements from groups have preferred numbers of valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom. Magnesium Valence Electrons Atom.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Magnesium Valence Electrons Atom For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. An atom in group 1 has only a single valence electron. 93 rows you may assume the valences of the chemical. Magnesium Valence Electrons Atom.
From www.alamy.com
3d render of atom structure of magnesium isolated over white background Magnesium Valence Electrons Atom Elements from groups have preferred numbers of valence electrons. 119 rows valence electrons: In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. An atom in group 1 has only a single valence electron. This one valence electron is easily lost to form a positive ion. Magnesium Valence Electrons Atom.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Magnesium Valence Electrons Atom Elements from groups have preferred numbers of valence electrons. An atom in group 1 has only a single valence electron. 119 rows valence electrons: In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron.. Magnesium Valence Electrons Atom.
From nursehub.com
Electron Shells NurseHub Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. This one valence electron is easily lost to form a positive ion with. Magnesium Valence Electrons Atom.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Valence electrons are the electrons that reside in the outermost. Magnesium Valence Electrons Atom.
From periodiske-system.dk
Magnesium Magnesium Valence Electrons Atom In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. An atom in group 1 has only a single valence electron. 93 rows you may assume the valences of the chemical. Magnesium Valence Electrons Atom.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Valence Electrons Atom Elements from groups have preferred numbers of valence electrons. An atom in group 1 has only a single valence electron. 119 rows valence electrons: For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Magnesium Valence Electrons Atom.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can. Magnesium Valence Electrons Atom.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. 93 rows you may assume the. Magnesium Valence Electrons Atom.
From mungfali.com
Magnesium Orbital Diagram Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. For example, alkali metal atoms (e.g., lithium, sodium) have one. Magnesium Valence Electrons Atom.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible. Magnesium Valence Electrons Atom.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Magnesium Valence Electrons Atom For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Elements from groups have preferred numbers of valence electrons. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a.. Magnesium Valence Electrons Atom.
From commons.wikimedia.org
FileElectron shell 012 Magnesium.svg Wikimedia Commons Magnesium Valence Electrons Atom 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. 119 rows valence electrons: Valence electrons are the electrons that reside in the outermost energy. Magnesium Valence Electrons Atom.
From www.youtube.com
Atomic Structure (Bohr Model) for Magnesium (Mg) YouTube Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. An atom in group 1 has only a single valence electron. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. In chemistry and physics, valence electrons are. Magnesium Valence Electrons Atom.
From valenceelectrons.com
Electron Configuration for Magnesium and ion(Mg2+) Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. An atom in group 1 has only a single valence electron. Valence electrons are the electrons that reside in the outermost energy level of. Magnesium Valence Electrons Atom.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Valence Electrons Atom Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This one valence electron is easily lost to form. Magnesium Valence Electrons Atom.
From www.slideshare.net
The periodic table Magnesium Valence Electrons Atom Elements from groups have preferred numbers of valence electrons. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds.. Magnesium Valence Electrons Atom.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Valence Electrons Atom 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Elements from groups have preferred numbers of valence electrons. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the. Magnesium Valence Electrons Atom.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Valence Electrons Atom 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. Elements from groups have preferred numbers of valence electrons. Valence electrons are the electrons that reside in the outermost energy. Magnesium Valence Electrons Atom.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Magnesium Valence Electrons Atom Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. This one valence electron is easily lost to form a positive ion. Magnesium Valence Electrons Atom.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Elements from groups have preferred numbers of valence electrons. In chemistry and physics, valence electrons are electrons in the outermost shell of. Magnesium Valence Electrons Atom.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Magnesium Valence Electrons Atom 119 rows valence electrons: This one valence electron is easily lost to form a positive ion with an #s^2p^6#. Elements from groups have preferred numbers of valence electrons. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in. Magnesium Valence Electrons Atom.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Magnesium Valence Electrons Atom.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Valence Electrons Atom Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. An atom in group 1 has only a single valence electron. This one valence electron is easily lost to form a. Magnesium Valence Electrons Atom.
From periodictable.me
Magnesium Valence Electron Magnesium Valency (Mg) with Dot Diagram Magnesium Valence Electrons Atom 119 rows valence electrons: In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Valence electrons are the electrons that reside in. Magnesium Valence Electrons Atom.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. Elements from groups have preferred numbers of valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. 119 rows valence electrons:. Magnesium Valence Electrons Atom.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Magnesium Valence Electrons Atom An atom in group 1 has only a single valence electron. Elements from groups have preferred numbers of valence electrons. 119 rows valence electrons: Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. For example, alkali metal atoms (e.g., lithium, sodium) have. Magnesium Valence Electrons Atom.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Valence Electrons Atom This one valence electron is easily lost to form a positive ion with an #s^2p^6#. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. 119 rows valence electrons: An atom in group 1. Magnesium Valence Electrons Atom.
From www.dreamstime.com
Atom Of Magnesium With Detailed Core And Its 12 Electrons Stock Magnesium Valence Electrons Atom For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. This one valence electron is easily lost to form a positive ion with an #s^2p^6#. In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. Valence electrons are the electrons that reside in the. Magnesium Valence Electrons Atom.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Magnesium Valence Electrons Atom In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a. An atom in group 1 has only a single valence electron. 119 rows valence electrons: This one valence electron is easily lost to form a positive ion with an #s^2p^6#. 93 rows you may assume the. Magnesium Valence Electrons Atom.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Magnesium Valence Electrons Atom Elements from groups have preferred numbers of valence electrons. Valence electrons are the electrons that reside in the outermost energy level of an atom and are, therefore, the most accessible for the formation of chemical bonds. For example, alkali metal atoms (e.g., lithium, sodium) have one valence electron. An atom in group 1 has only a single valence electron. 119. Magnesium Valence Electrons Atom.